Scientific Achievement
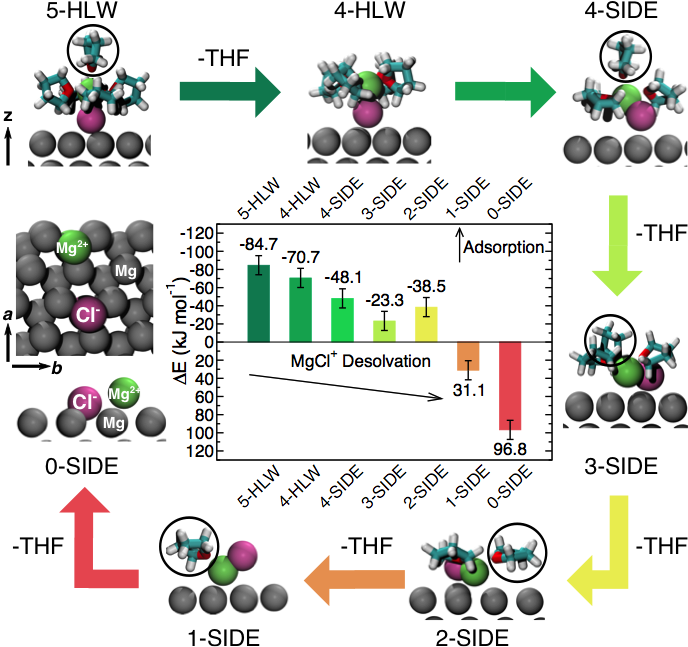
The chemical species at the Mg-anode surface in the presence of Magnesium Aluminum-Chloro complex (MACC) electrolyte were identified. While solvent molecules (THF and DME) are loosely bound at the Mg(0001) surface, the constituents of the MACC electrolyte (e.g. MgCl+, see diagram) are strongly anchored at the electrode.
Significance and Impact
The study demonstrates why Mg deposition is possible in the MACC electrolyte, unlocking the preliminary desolvation steps before the electrode transfer occurs. Simulations also suggest that the solvent does not passivate the surface of the anode electrode.
Research Details
- Modeling based on first principles DFT and ab initio molecular dynamic are used to clarify the structure of the Mg anode surface.
- The model derived from DFT adsorption energies and dynamical properties of the Mg salts at the surface suggests small desolvation energies (~60 kJ mol-1 )of Mg2+ ions during plating.
Work performed at Massachusetts Institute of Technology (JCESR collaborator) , Lawrence Berkley National Laboratory (JCESR partner), and Sandia National Laboratory (JCESR partner) by P. Canepa, G. Sai Gautam, R. Malik, S. Jayaraman, Z. Rong, K. R. Zavadil, K. Persson and G. Ceder, Chem. Mater., 2015.

