Scientific Achievement
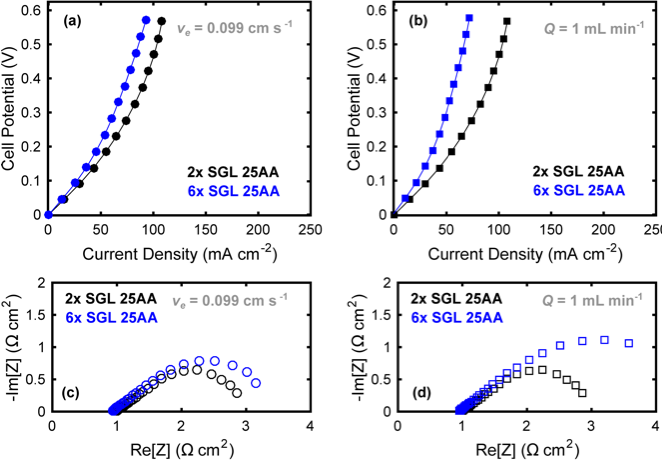
Sources of resistive losses were experimentally minimized for a nonaqueous flow cell for various active species concentrations, electrolyte compositions, flow rates, separators, and electrode thicknesses.
Significance and Impact
This work experimentally demonstrates that, with appropriate cell engineering, nonaqueous flow cells can achieve an area specific resistance as low as 1.7 Ω cm2, validating their potential as cost-competitive energy storage systems.
Research Details
- A model redox active species, Fc1N112+/2+, was employed in both acetonitrile- and propylene carbonate-based electrolytes.
- Single electrolyte flow cells (2.55 cm2 & 25 cm2) were used to demonstrate performance and scalability through polarization and electrochemical impedance spectroscopy.
- The influence of flowrate, separator thickness, electrolyte composition, and electrode thickness were explored.

