Scientific Achievement
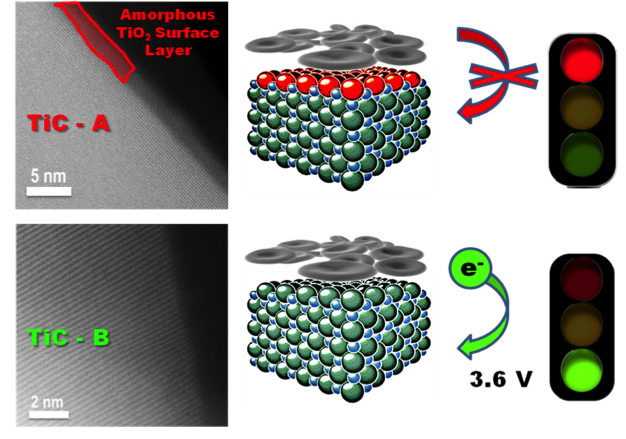
Identification of the critical role the surface properties of metallic TiC & TiN supports play in Li-O2 electrochemistry. Oxygen evolution from bulk Li2O2 is enabled by “clean” surfaces but completely inhibited by 2-3 nm thick TiO2 films.
Significance and Impact
- Electron transport through insulating surface layers governs the overpotential for the O2 evolution reaction
- Although bulk conductivity of the support for the oxygen reduction reaction is required, control of the surface chemistry at the nanoscale is critical for high performing electrodes
- The ability to completely oxidize sub-micron Li2O2 particles at a flat, relatively low potential suggests OER does not require electrocatalysis
Research Details
- Electrodes prefilled with 200-800 nm Li2O2 crystallites were compared with electrochemically deposited lithium peroxide
- A combination of XPS, on-line mass spectrometry, S/TEM, and electrochemical methods to probe the nature of surface films on carbon and conductive Ti-based nanoparticles enabled this research
Work performed at University of Waterloo (JCESR collaborator) and Pacific Northwest National Laboratory (JCESR partner) Brian Adams, Robert Black, Claudio Radtke, Zack Williams, B. Layla Mehdi, Nigel Browning and Linda Nazar ACS Nano, 2014, 8 (12), 2483–12493

