Scientific Achievement
A new model of electrodeposition was developed that includes crucial phenomena required to simulate Mg battery anode cycling and yields results that are consistent with experimental observations.
Significance and Impact
This work provides insight into the role of the electrolyte concentration near a Mg deposit and of the orientation dependence of the reaction rate on the morphology of the anode. This model also provides a platform to investigate the origin of observed morphologies and to determine conditions for improved performance.
Research Details
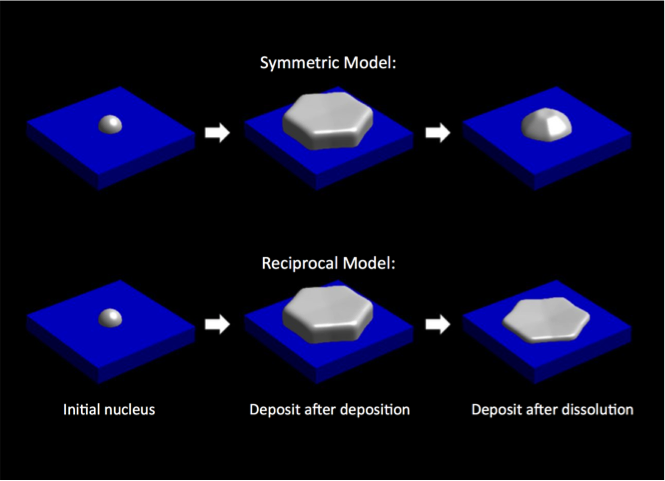
- Model captures Butler-Volmer kinetics, faceted growth/dissolution, and spatial distribution and temporal evolution of the potential and concentration.
- Scanning electron microscopy (SEM) of Mg deposits revealed a faceted hexagonal prism morphology.
- Simulations revealed substantial depletion of the electrolyte near the deposit, providing evidence that electrolyte depletion may suppresses deposit growth and favor nucleation elsewhere.
- Two models for the orientation dependence of the reaction rates were investigated, providing predictions of the deposit morphology for two possible dominant mechanisms.
Work performed at University of Michigan (JCESR partner) and Sandia National Laboratories (JCESR partner) by S. DeWitt, N. Hahn, K. Zavadil, and K. Thornton, J. Electrochem. Soc., 2016, 163 (3), A513-A521

