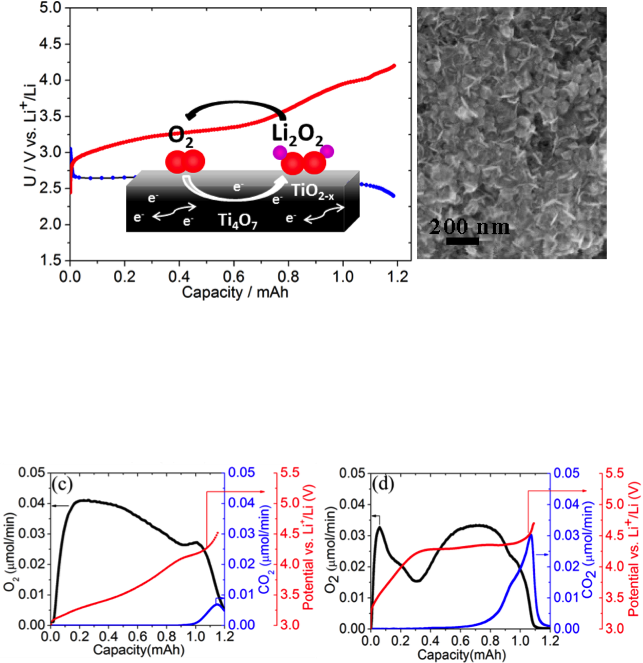
(Top Left) SEM image shows Li2O2 as ~10 nm thin platelets and film formed on Ti4O7 cathode.
(Bottom) On-line mass spec analysis of gas evolution from the Ti4O7 cell (left) compared to a Vulcan carbon cell (right). The red, black and the blue curves depict voltage variation, O2 evolution, and CO2 evolution during galvanostatic charge.
Scientific Achievement
- Synthesis of a low-cost nanocrystalline metallic metal oxide that shows very good ORR/OER properties in a Li-O2 cell, highlighting the importance of controlling the cathode interface to achieve better round-trip efficiency
- Demonstration of the presence of a conductive, self-passivating sub-stoichiometric metal oxide layer formed at the surface, which is important for stability
Significance and Impact
- First report of ultra high surface area (180 m2/g) Ti4O7
- One of the few non-carbonaceous ORR/OER cathodes reported to date (aside from Au and TiC) that overcomes the problem of insulating lithium carbonate formed at carbon interfaces on reaction with Li2O2
- The stable and conductive interface greatly reduces the overpotential compared to carbon: oxygen evolution starts at 3.0V and the majority of O2 release occurs in the 3.0-3.5V window, very comparable to Au
Research Details
- Single phase nanocrystalline (10-20 nm) Ti4O7 was prepared by a one-step carbothermal reduction at 870⁰ C
- A combination of XPS, on-line mass spectrometry, HRTEM, XRD and electrochemical methods were used to identify the Magnéli phase, the nature of the peroxide particles, and the interface formed between them on discharge
Work performed at the University of Waterloo (JCESR Collaborator) Dipan Kundu, Robert Black and Linda Nazar Energy & Environmental Science, 2015,
DOI: 10.1039/C4EE02587C

