Scientific Achievement
- Ionic liquids (ILs) have wide electrochemical stability windows, low vapor pressures, and low flammabilities, making them attractive as replacements for conventional organic solvents like THF and DME
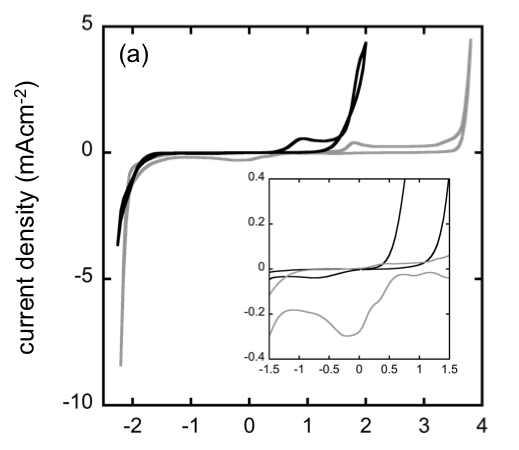
- The electrochemistry of several Mg salts in room-temperature ILs was studied using plating/stripping voltammetry to assess the viability of IL solvents for applications in secondary Mg batteries
Significance and Impact
- Contrary to some prior reports, no salt/IL combination appeared to facilitate reversible Mg plating
- The absence of voltammetric signatures of Mg plating from ILs with Tf2N− and BF4− suggests that strong Mg/anion Coulombic attraction inhibits electrodeposition
- The results highlight the need for IL solvents or cosolvent systems that promote Mg2+ dissociation
Research Details
- Borohydride (BH4−), trifluoromethanesulfonate (TfO−), and bis(trifluoromethanesulfonyl)imide (Tf2N−) salts of Mg were investigated. Three ILs were considered: l-n-butyl-3-methylimidazolium (BMIM)-Tf2N, N-methyl-N-propylpiperidinium (PP13)-Tf2N, and N,N-diethyl-N-methyl(2-methoxyethyl)-ammonium (DEME+) tetrafluoroborate (BF4−).
- Salts and ILs were combined to produce binary solutions in which the anions were structurally similar or identical, if possible
Work performed at the University of Michigan (JCESR partner) by G. Vardar, A. E. S. Sleightholme, J. Naruse, H. Hiramatsu, D. J. Siegel, ACS Applied Materials & Interfaces, 2014.
DOI: 10.1021/am5049064

