Scientific Achievement
Building on prior collaborative JCESR research on iteratively advancing phenothiazines as charge storage materials, we developed a new class of phenothiazine derivatives with high equivalent charge concentration by increasing solubility and stability of multiple electron transfer events.
Significance and Impact
Two-electron-donating phenothiazines with oligoglycol substituents exhibit high solubility across all states of charge. The radical cation form is sufficiently stable that it can be isolated in as a crystalline solid. Lessons learned from this activity may, in turn, inform future design strategies for phenothiazines and may be applicable to other molecular families.
Research Details
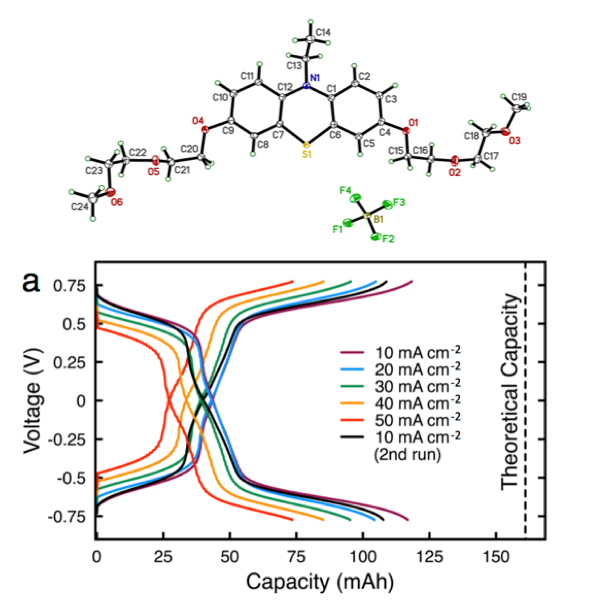
- The new phenothiazine derivative is stable across three states of charge, with a limiting solubility of 0.55 M in non-aqueous electrolytes, enabling solutions with capacity of >1 M e-.
- Symmetric flow cell cycling demonstrates stability over multiple discharge / charge cycles with only 30% capacity fade over 460 h of operation with an electrolytes containing 0.3 M active material.
- Post-test analysis indicates performance decay is due to self-discharge and crossover, not materials decomposition.

