Scientific Achievement
A new method that allows in situ, real-time monitoring of dissolution rates of transition metal cations from oxide cathode hosts, as well as monitoring of deintercalation of the working cation (Li, Mg, Zn, etc.) simultaneously with electrochemical cycling.
Significance and Impact
This unique method enables independent evaluation of the stability of transition metal oxide host materials with part-per-billion-level sensitivity under realistic cycling conditions.
Research Details
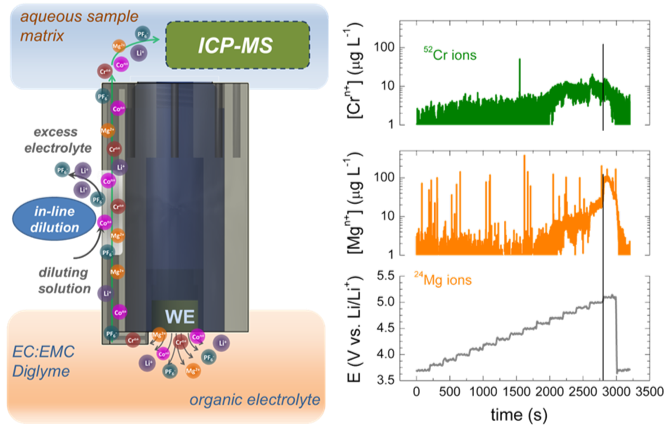
- Coupling of the SPRDE with an inductively coupled plasma mass spectrometer (ICP-MS) enables analysis of almost any element in the periodic table, providing significant versatility in studying beyond-lithium-ion battery chemistries.
- By cycling a Mg cathode in a lithium electrolyte, it was possible to monitor dissolution of both Cr and Mg from the high voltage MgCr2O4 spinel cathode, showing significantly greater Mg dissolution than that of Cr and demonstrating effective deintercalation of Mg from this material.
- SPRDE-ICPMS can serve as a test bed for candidate high voltage cathode materials that are difficult to evaluate under normal coin cell testing due the lack of stable electrolytes at high voltages vs. Mg.

