Scientific Achievement
- First-principles analysis of the screening of Mg2+ ions upon intercalation within metallic Mo6S8
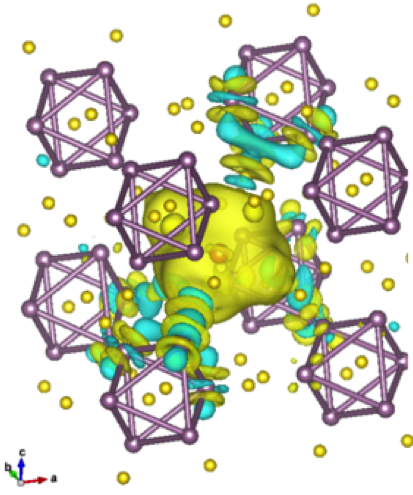
- Observed complete screening of intercalate Mg2+ ions on the length scale of one unit cell (~6Å)
- Mo6 octahedra are not the primary redox unit for intercalation (as previously believed) – screening is localized around the Mg2+ ion and neighboring S atoms
Significance and Impact
- Provides atomic level understanding of electronic and ionic behavior of metallic intercalation cathodes for multivalent-ion batteries, promising candidates for next-generation beyond lithium-ion batteries
- Fast electroneutrality achievable in metallic systems via band conduction rather than polaronic mechanisms
- Localized screening effectively removes cation-cation repulsion as a bottleneck to diffusion
- Shift in design focus away from transition metal clusters towards cavity geometry and anion binding as the primary mediators of intercalation phenomena
Research Details
- First-principles analysis based on Density Functional Theory using the plane-wave pseudopotential formalism, GGA(PBE)+U
- Atom-centered electron populations determined using Bader analysis
Work performed at Lawrence Berkeley National Laboratory’s Molecular Foundry using NERSC supercomputing facilities. Thoele and Prendergast “Strongly localized electronic screening of Mg intercalants in the Chevrel Phases Mo6S8” (in preparation).

