Scientific Achievement
- The precise decomposition pathways of glyme solvents were identified.
- Some of the limitations (ionic conductivity, viscosity) of the novel electrolyte provide a step forward in design strategies for other stable electrolytes.
Significance and Impact
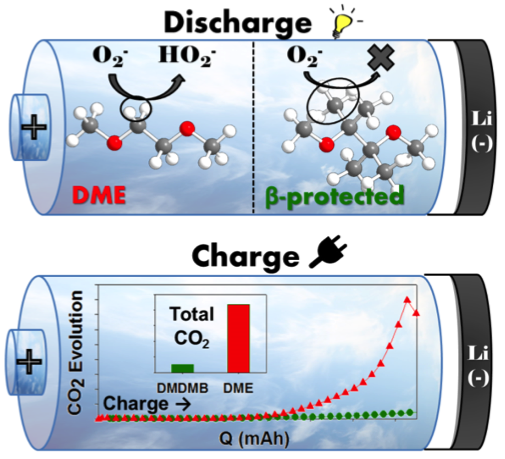
- Protection of the β-hydrogens in the commonly used DME electrolyte results in a solvent DMDMB with enhanced stability toward hydrogen abstaction by superoxide.
- Significantly decreased amounts of decomposition products with DMDMB during discharge
- Approximately 10x less CO2 evolution during charge with DMDMB
Research Details
- DMDMB was synthesized and purified. The addition of LiTFSI salt resulted in a novel chelate ionic liquid electrolyte.
- A combination of NMR, online mass spectrometry, XRD, and electrochemical methods were used to analyze the side products formed in Li-O2 batteries using the [(DMDMB)2Li]TFSI and [(DME)2Li]TFSI electrolytes.
Work performed at the University of Waterloo (JCESR collaborator) and the Paul Scherrer Institute (Switzerland) by Brian Adams, Robert Black, Zack Williams, Russel Fernandes, Marine Cuisinier, Erik Jaemstorp Berg, Petr Novak, Graham Murphy, and Linda Nazar Advanced Energy Materials, 2015.

