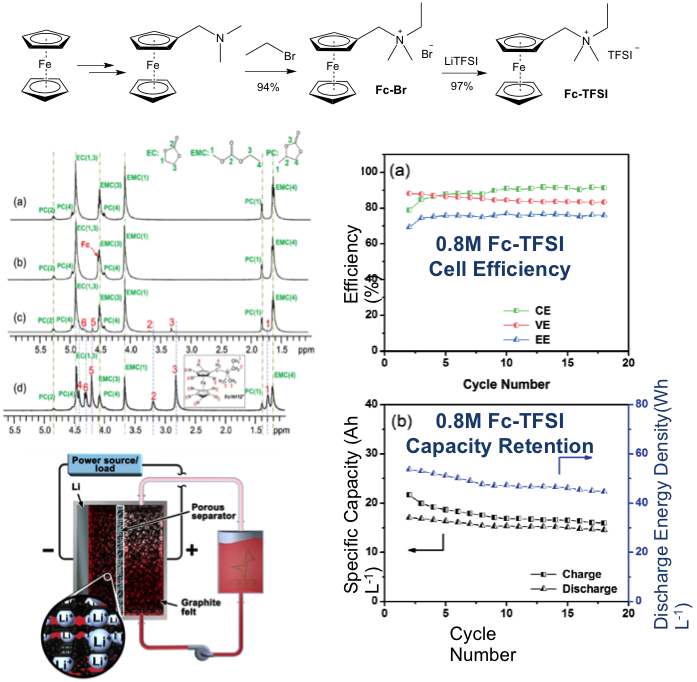
(Left) NMR Decoding Solvation
(Right) Li-Graphite Hybrid Anode Decent cyclability at high conc.
Scientific Achievement
Material tailoring led to a significant increase in the solubility of the ferrocene redox material. NMR measurement was able to decode the solvation mechanism and explain the solubility increase of the tailored compound. A Li-graphite hybrid anode enabled the nonaqueous Li/organic cell design to produce decent cyclability at high redox material concentrations and deliver energy density of ~45 Wh/L.
Significance and Impact
Rational molecular engineering is viable to improve solubility of redox materials. High-conc cycling is challenging for nonaqueous flow chemistry, but improved cell design can help.
Research Details
- NMR measurements were performed to help understand the molecule-solvent interactions.
- Flow cell cycling at various conditions was carried out to determine the performance-limiting factors: redox material concentration, electrolyte additive, and hybrid anode design.
Work performed at Pacific Northwest National Laboratory (JCESR partner) by X. Wei, L. Cosimbescu, W. Xu*, J.Z. Hu, M. Vijayakumar, J. Feng, M.Y. Hu, X. Deng, J. Xiao, J. Liu, V. Sprenkle and W. Wang*, Adv. Energy Mater. 2015.

