Scientific Achievement
A series of alkoxide/siloxide magnesium electrolytes were developed with environmentally benign MgCl2 and ROMgCl (R=alkyl or silyl). The resulting electrolytes possess oxidative stability up to about 3.5 V (vs. Mg/Mg2+), representing one of the best stability performing among all reported electrolytes for Mg-ion batteries. The mechanistic study towards the generation of active ionic species was extensively carried out.
Significance and Impact
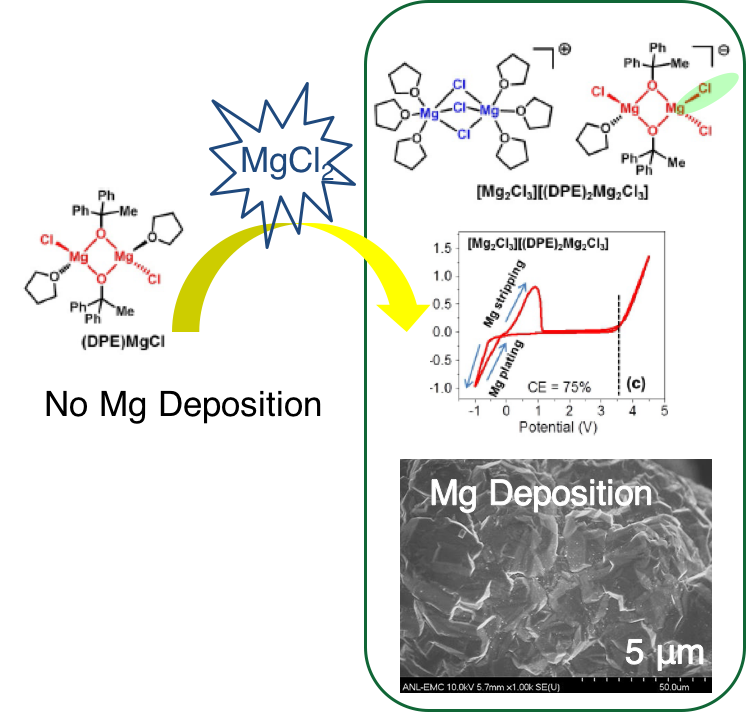
Instead of using corrosive and toxic strong Lewis acid AlCl3, environmentally benign MgCl2 has been used and both Mg deposition and high anodic stability was obtained for the ROMgCl-MgCl2 mixture. The equilibrium was established unambiguously and MgCl2 played a pivotal role.
Research Details
- Addition of benign salt MgCl2 into (DPE)MgCl in THF solution enable the electrolyte to show both deposition and high anodic stability . The excellent battery cycling performance of DPEMgCl-MgCl2 using Chevrel phase Mo6S8 further demonstrates the great potential of our electrolyte for rechargeable magnesium-ion batteries.
Work performed at Argonne National Laboratory (JCESR managing partner) by B. Pan, J. Huang, M. He, S. Brombosz, J. Vaughey, L. Zhang, A. Burrell, Z. Zhang, and C. Liao, ChemSusChem, 2016, 9, 595-599.

