Scientific Achievement
Demonstrated a strategy for expanding the electrochemical stability window for magnesium batteries through predictive anion design while discovering new mechanisms of interphase formation.
Significance and Impact
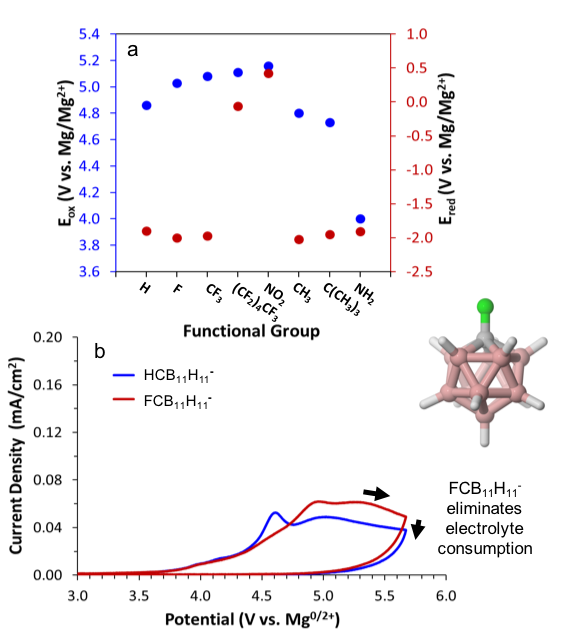
The EES community lacks knowledge of and design strategies for the synthesis of sufficiently stable Mg2+ electrolytes and protective electrode interphases. This accomplishment elucidates a pathway for the rational stabilization of multivalent electrolyte anions via electron-withdrawing-group functionalization. The discovery of unique film formation suggests a strategy for ensuring interfacial stability during charge transfer through rational design of cathode interphases.
Research Details
Computational prediction and experimental validation of electrolyte reduction and oxidation stability, speciation, and conductivity.
Quantitative determination of the role of anion derivatization in electrolyte stability and speciation, defining a path for tailored solvation of Mg2+.
Unique film formation route defines a path for superionic Mg2+ transport in tailored solid-electrolyte-interphases for high voltage MV-cathode protection.

