Scientific Achievement
Mechanistic predictions for the structure and molecular conversion pathways in the discharge reaction LiO2–> Li2O2 in Li-O2 batteries were made using high level quantum chemical theory.
Significance and Impact
Provides essential scientific understanding for optimizing the energy and reversibility of the Li-O2 system, which potentially has 10 times the energy density of Li-ion batteries.
Research Details
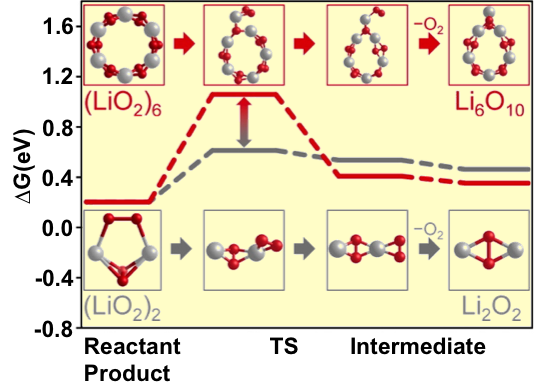
- The stability of planar superoxide (LiO2)n rings was calculated to increase up to n=12, after which 3-D stacked ring structures containing elements of the bulk LiO2 structure become more stable.
- Barriers for conversion of (LiO2)n to peroxide (Li2O2) are higher for larger clusters (higher values of n), slowing the conversion rate and possibly allowing a mixed lithium superoxide/lithium peroxide final reaction product.
- Previous experiments demonstrated that such a mixed LiO2/Li2O2 reaction product increased the efficiency of the system, critical for making these batteries viable.
Work performed at Argonne National Laboratory, JCESR managing partner. U. Das, K.C. Lau, P.C. Redfern and L.A. Curtiss, Structure and Stability of Lithium Superoxide Clusters and Relevance to Li–O2 Batteries, J. Phys. Chem. Lett., 2014, 5, pp 813–819.
DOI: 10.1021/jz500084e

