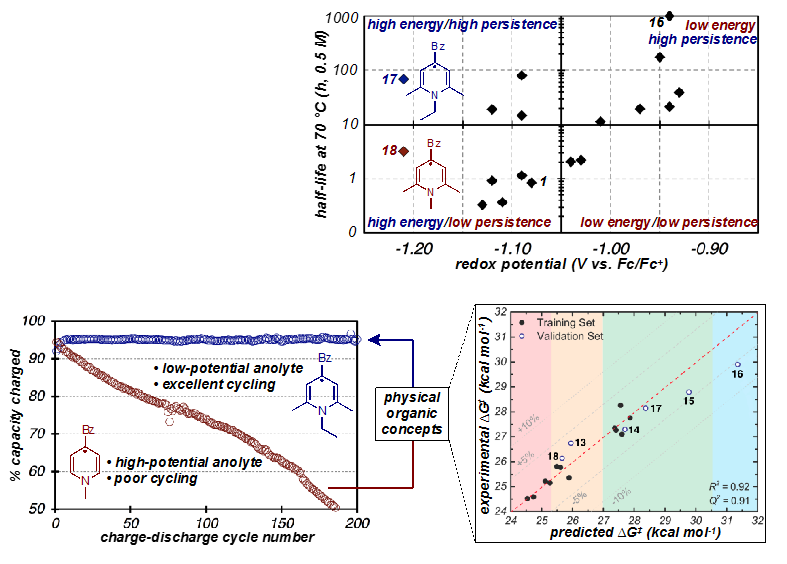
(Bottom, Left) Improvement in charge-discharge cycling performance between the first generation anolyte and the designer anolyte that was identified by predictive multidimensional analysis.
(Bottom, right) Plot demonstrating the excellent agreement between predicted stability of charged anolytes and experimental measurements over a range of seconds to months.
Scientific Achievement
- A rare example of a high energy (low potential) anolyte with excellent stability in the charged state was identified. This material undergoes cycling at potentials lower than other studied materials but does not show degradation even after 200 charge-discharge cycles.
- Predictive multidimensional analysis, a technique common to physical organic chemistry, was successfully applied to the development of a model for RFB electrolytes that predicts their stability in the charged state with high accuracy.
Significance and Impact
- While this study identifies a stable, low potential anolyte, its greatest significance and impact is the demonstration of a powerful new strategy that is broadly applicable to the development of any class of RFB electrolytes.
Research Details
- Kinetic analysis suggests that charged anolytes decompose through dimerization pathways.
- Models suggest that substituent height above the pyridine plane is a key contributor to stability.
Work was performed at the University of Michigan (JCESR partner) and University of Utah (JCESR funded collaborator) by C. S. Sevov, D. P. Hickey, S. G. Robinson, S. D. Minteer, M. S. Sigman, and M. S. Sanford. Journal of the American Chemical Society, 2017, 139, 2924.

