Scientific Achievement
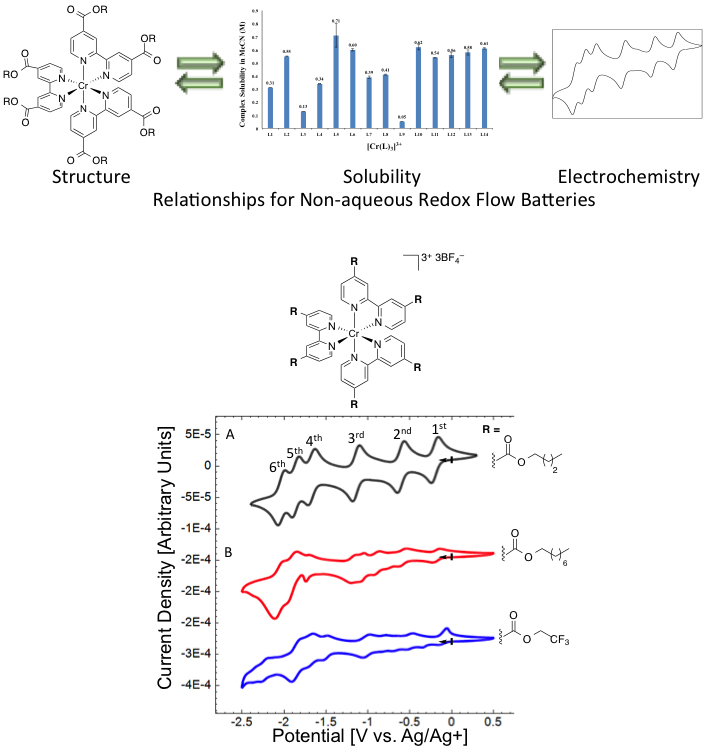
Synthesis of 24 Cr-bipyridine complexes across two different oxidation states; their solubility varies from insoluble to 0.7 M. Most of the complexes undergo reversible electrochemistry demonstrating up to six reversible redox couples.
Significance and Impact
Major differences in solubility are observed (up to 4 orders of magnitude) between active species bearing the same ligands but at different oxidation states. These solubility variations are attributed mostly to the ionic differences in the complexes (Cr3+ versus Cr0). Modifications to the metal complex ligand backbone by functional groups and electronic effects can significantly impact the stability of the complexes, number of accessible redox couples, and cell voltage.
Research Details
- Synthesis of Cr-complexes and characterization by high resolution mass spectrometry and elemental analysis.
- Metal complexes solubility determination by UV-vis absorption spectroscopy and electrochemical characterization by cyclic voltammetry (CV)
Work was performed at the University of Michigan, Department of Chemistry and Department of Chemical Engineering (JCESR Partner) by P. J. Cabrera, X. Yang, J. A. Suttil, R. E. M. Brooner, L. T. Thompson and M. S. Sanford, Inorganic Chemistry, 2015, 54, 10214.

