Scientific Achievement
A highly collaborative team of theorists and experimentalists identified a nanostructured redox mediator for soluble polysulfides, synthesized the target compound, electrochemically validate the computations, and demonstrated enhanced current in redox mediated Li-S cells.
Significance and Impact
Overcoming charge-transport bottlenecks in Li-S cells brings a viable prototype for both transportation and grid closer to reality as improved current density enables the high power operation required for transportation and decreases overall component costs necessary for grid applications.
Research Details
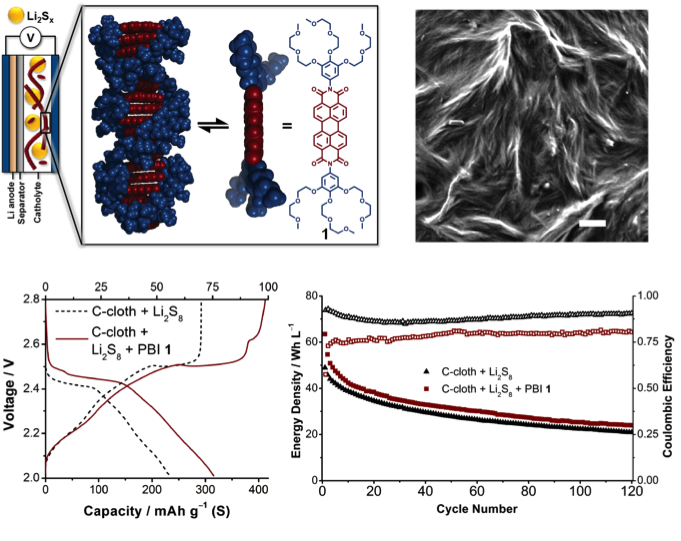
- Candidate redox mediators were screened by electron affinity with a high-throughput computational platform developed for large molecules with up to 200 atoms.
- Perylene bisimide (PBI) was identified as an excellent match for the redox potential of soluble polysulfides (2.5 V vs. Li/Li+), and PBI redox mediators were synthesized that organized into 1D-nanowire aggregates to move charge via self-exchange and hopping in addition to diffusion.
- Sulfur utilization was improved by >30 percent in the soluble polysulfide regime with nanostructured PBI mediators.
Work performed at Lawrence Berkeley National Lab (JCESR Partner) and Massachusetts Institute of Technology (JCESR Collaborator) by P.D. Frischmann, L.C.H. Gerber, S.E. Doris, E.Y. Tsai, F.Y. Fan, X. Qu, A. Jain, K. A. Persson, Y.-M. Chiang and B.A. Helms

