Scientific Achievement
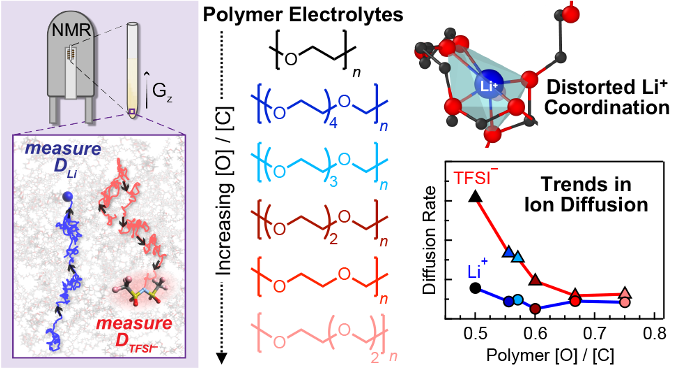
Polyacetal electrolytes can electrochemically outperform the well-established poly(ethylene oxide) systems used in Li-ion batteries. In this work, we employ a suite of experimental and theoretical techniques to reveal the underlying molecular interactions between cations, anions and the polyacetal host that lead to improved electrochemical efficacy.
Significance and Impact
Enhanced ion transport in polyacetals for Li-ion battery applications is ascribed to the following factors: (1) greater diversity of cation–polymer coordination environments, leading to faster cation diffusivity, and (2) formation of anion-rich clusters, leading to slower anion diffusivity.
Research Details
- 19F PFG measurements show anion self-diffusion slows as polymer oxygen content increases; Raman spectroscopy confirms clustering of anions. Cation diffusivity probed by 7Li PFG is correlated with MD simulations.
The most efficacious electrolytes, denoted P(EO-MO) and P(EO-2MO), possess the slowest anion self-diffusion

