Scientific Achievement
The electrochemical behavior of MgM2S4 (M = Ti, Cr) thiospinels was investigated experimentally and their redox activity was understood through analysis of the computed electronic density of states.
Significance and Impact
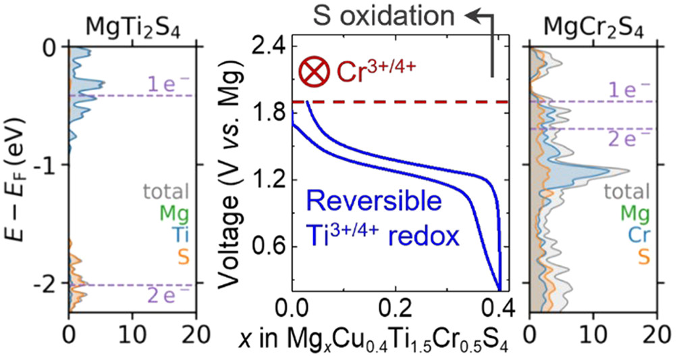
Previous computational screening led to the identification of MgCr2S4 as a candidate high-voltage cathode material for Mg batteries, but Mg2+ extraction from this material was found to be prohibited by coupling of Cr and S electronic states near the Fermi energy.
Research Details
- MgCr2S4 and Cu0.4Ti1.5Cr0.5S4 samples were synthesized and electrochemically evaluated for Mg2+ extraction and insertion, respectively.
- MgCr2S4 was shown to be electrochemically inactive, and Cu0.4Ti1.5Cr0.5S4 was shown to uptake ~0.4 mol Mg2+ at
~1.3 V, corresponding with only Ti and not Cr oxidation. - Density functional theory (DFT) calculations identify the origin of the electrochemical inactivity of Cr, and a calculable descriptor for redox activity was proposed to improve future surveys of cathode candidates.

