Scientific Achievement
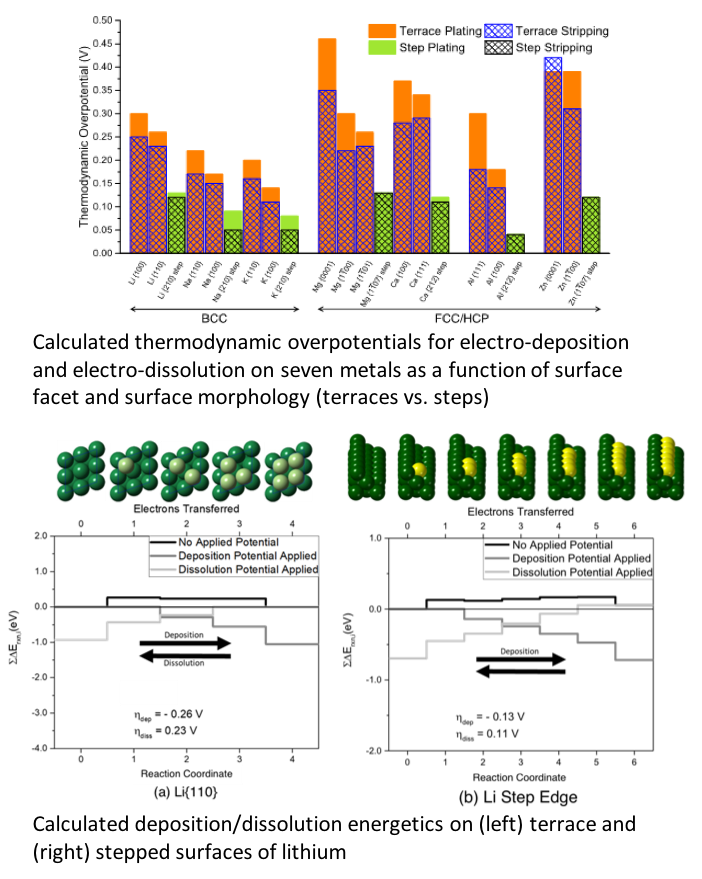
Thermodynamic contributions to overpotentials are predicted for seven metals relevant for next-generation metal anode batteries: Al, Ca, K, Li, Mg, Na, and Zn. In addition, a multi-scale model that combines these predictions with nucleation theory is used to estimate nucleation rates for electrodeposits on these electrodes.
Significance and Impact
To be viable, the plating and stripping of metal ions at the anode must occur with low overpotentials. The present study systematically quantifies these efficiency factors across several metals and as a function of surface morphology.
Research Details
- The size of the overpotential for a given metal is observed to correlate with the difference between the coordination number for a surface atom and that of an atom in the bulk.
- Overpotentials are predicted to be smallest at steps, and largest at terrace sites.
- Body-centered alkali metals (Li, Na, and K) are predicted to be among the most efficient (lowest overpotentials); in contrast, Al, Zn, Ca, and Mg exhibit higher overpotentials due to their larger bulk coordination.

