Scientific Achievement
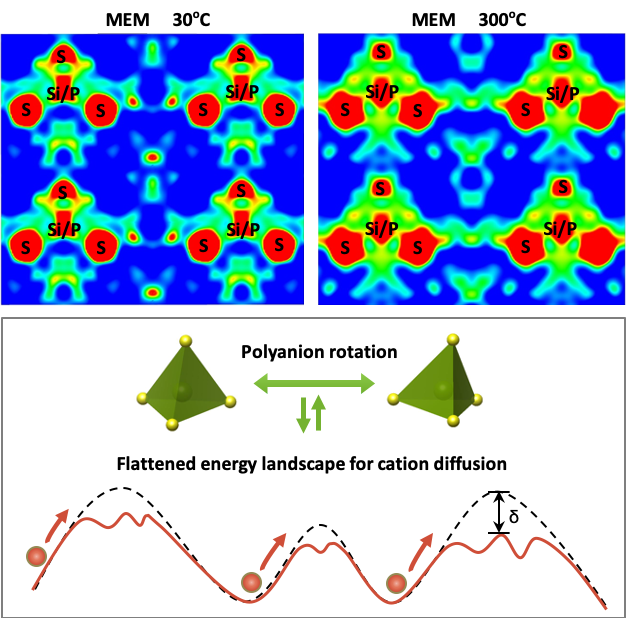
Using the maximum entropy method and AIMD calculations, we demonstrate that both the high-temperature phase, b-Li3PS4, and its room temperature Li-stuffed counterpart, Li3.25Si0.25P0.75S4 exhibit anion rotation which increases Li-cation diffusivity.
Significance and Impact
Previous studies mainly focussed on how the static crystalline structure affects Li-cation mobility, but potential impact from anion dynamics has been largely neglected. Here, we reveal that anion dynamics can be manipulated by the entropy-driven stabilization of a high temperature rotor phase to room temperature, and show that polyanion rotational motion in lithium thiophosphates is coupled to – and enhances – Li-ion diffusion.
Research Details
- (Si4++Li+) substitution for P5+ stabilizes the high temperature rotor phase (β-Li3PS4) owing to increased entropy. As a result, anion rotation persists down to room temperature, as evidenced from MEM plots, enhancing Li+-ion conductivity by a factor of 1000.
- AIMD studies confirmed anion rotational dynamics in both β-Li3PS4 and Si substituted Li3.25Si0.25P0.75S4 and reveals the low Helmholtz free energy barrier for this process.
- Joint-time correlation analysis revealed that the anion rotation is dynamically coupled to and facilitates the cation migration via the paddle-wheel mechanism by transiently widening the bottleneck for Li+-ion transport.

