Scientific Achievement
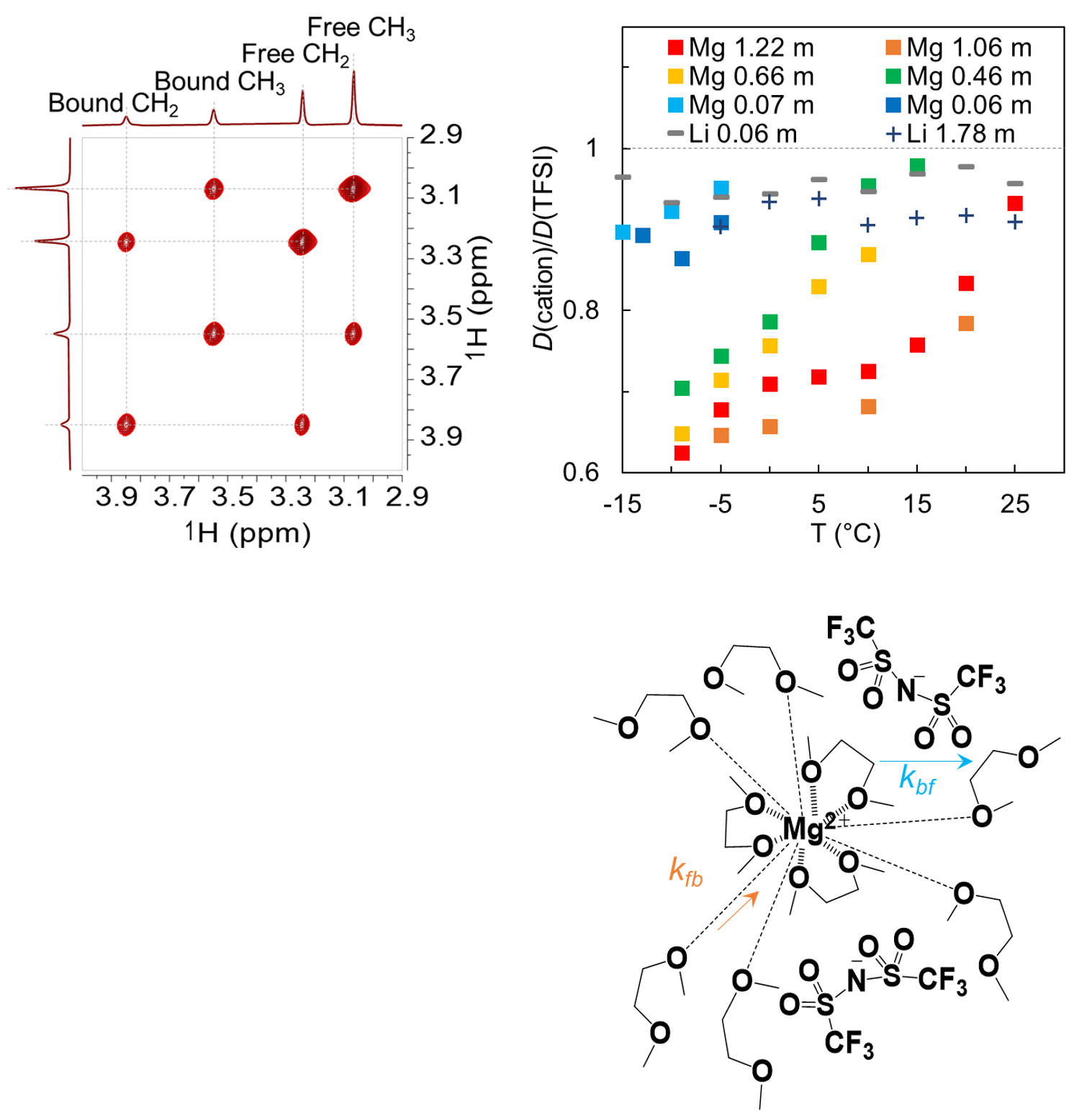
Comparison of the diffusion behaviors of Mg2+, TFSI-, DME, and Li+ reveals a relative restriction to Mg2+ diffusion that is caused by the long-range interaction between Mg2+ and solvent molecules, especially those with suppressed motions at high concentrations and low temperatures.
Significance and Impact
In order to reduce this long-range effect of multivalent ions and to increase their diffusivities in electrolytes (and crucially at interfaces within working cells), adding anion ligands or using a cosolvent system must be considered.
Research Details
- Exchange rates between “bound” DME (coordinating to Mg2+) and “free” DME (bulk) are obtained using 2D 1H-1H EXSY spectra.
- Self-diffusivities of free DME and bound DME (which is equal to that of Mg2+) before the exchange occurs are calculated using PFG diffusion NMR measurements coupled with analytical formulas describing diffusion under the condition of two-site exchange.
- The high activation enthalpy for exchange and the long lifetime of Mg-DME clusters can be explained by the structural change of bound DME as evidenced by its shorter C-H bond length manifested in 1H and 13C NMR.

