Scientific Achievement
The relative contributions of Zn2+ and H+ insertion to the overall capacity
of VPO4F are differentiated and quantified in aqueous and hybrid electrolytes. Increasing the salt concentration and/or proportion of nonaqueous solvent successfully suppresses water dynamics, improving the anodic stability and promoting Zn2+ intercalation over competing processes.
Significance and Impact
To be implemented in commercial applications (e.g. grid storage), Zn-ion technology must improve its low-rate and/or long-term electrochemical performance, which is often diminished by competing H+ intercalation-water oxidation mechanisms. Here, a hybrid aqueous-nonaqueous electrolyte was designed to tune interfacial dynamics and favor Zn2+ insertion, which increases the energy density and improves the cyclability
of VPO4F at slow rates.
Research Details
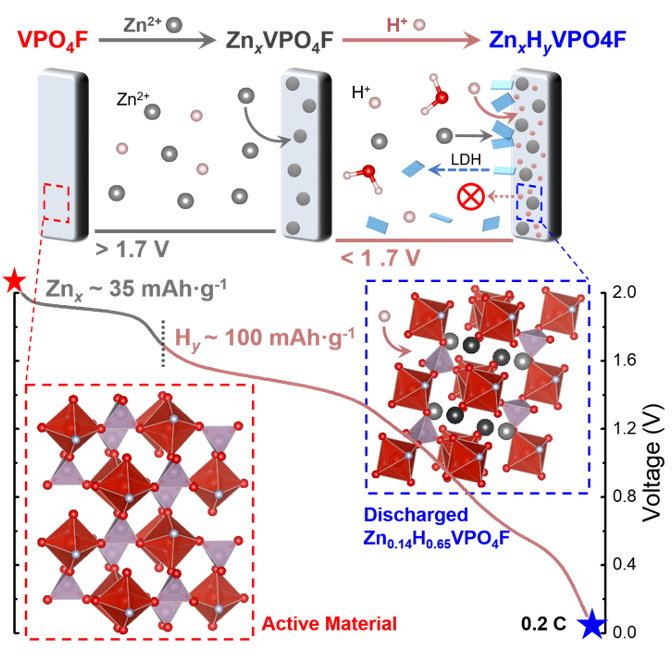
- The first successful demonstration of reversibile (de)insertion of a divalent ion in the high-voltage VPO4F tavorite structure was achieved.
- The Zn2+/H+ co-insertion processes taking place during discharge of VPO4F in aqueous electrolytes were differentiated and quantified, where high-voltage activity resulted from Zn2+ insertion.
- Similar to Li+ electrochemistry, two distinct phase transitions were observed upon Zn2+ insertion, depending on the degree of zincation.
- By employing a hybrid electrolyte, the electrochemical activity of water was tailored to disfavor H+ intercalation and improve anodic stability.
- When Zn2+ dominates electrochemistry, stable cycling at slow rates was observed and a high energy density of 237 Wh·kg-1 was attained.

