Scientific Achievement
The origin of strong acidity and explanation of ion diffusion along nanometric channels were uncovered in an important family of LiTFSI/H2O water-in-salt electrolytes (WiSEs).
Significance and Impact
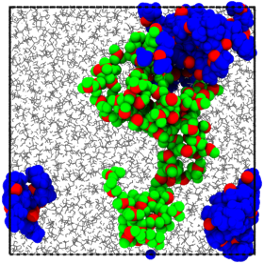
Structural similarities were discovered on the nanometer scale between LiTFSI/H2O WiSEs and hydrated Nafion® membranes, suggesting that Li+ ions in these WiSEs diffuse along complex pathways involving both water- and TFSI- rich regimes.
Research Details
- DFT calculations suggest that the origin of the unusual acidity of LiTFSI based WiSEs (e.g., a pH of of 2.4 at 20 m concentration) is due to the deprotonation of water molecules bridged over at least two Li+ ions as part of the multimeric salt structures within anion-rich regimes.
- PFG-NMR experiments and MD simulations disclose the phase separation of water- and TFSI- rich regimes and the existence of interfacial water between these two regimes.
- NMR spectroscopy and PFG-NMR measurements reveal the structural similarity of water-in-salt electrolyte with hydrated Nafion® membrane on the nanometer scale.

