Scientific Achievement
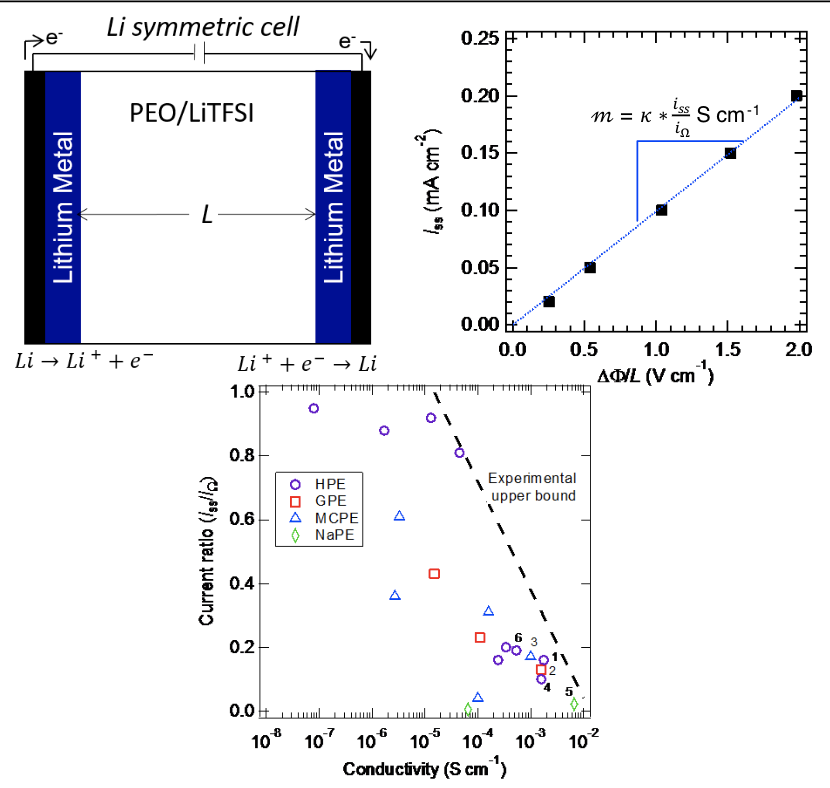
Battery electrolytes contain two mobile charged species of opposite charge and thus the traditional Ohm’s law, which applies to one mobile charged species, must be modified. This review uses a modified Ohm’s law to rank order electrolytes.
Significance and Impact
In the limit of low applied potentials, the current passed through an electrolyte is determined by two parameters: conductivity (κ) and a parameter that we call the current ratio. The highly-ranked electrolytes are characterized by low current ratio. Improving this parameter without sacrificing conductivity is a challenging but worthwhile goal.
Research Details
- Published conductivity and current ratio values were aggregated for a variety of electrolytes: homopolymer electrolytes (HPE), gel polymer or cross-linked electrolytes (GPE), multicomponent polymer electrolytes (MCPE), and polymer electrolytes containing a sodium salt (NaPE). All electrolytes, excluding NaPE, contained a lithium salt.
- Electrolytes developed thus far are limited by a trade-off between conductivity and current ratio leading to an upper bound that is similar to the permeability-selectivity upper bound that has been found in gas permeation membranes.

