Scientific Achievement
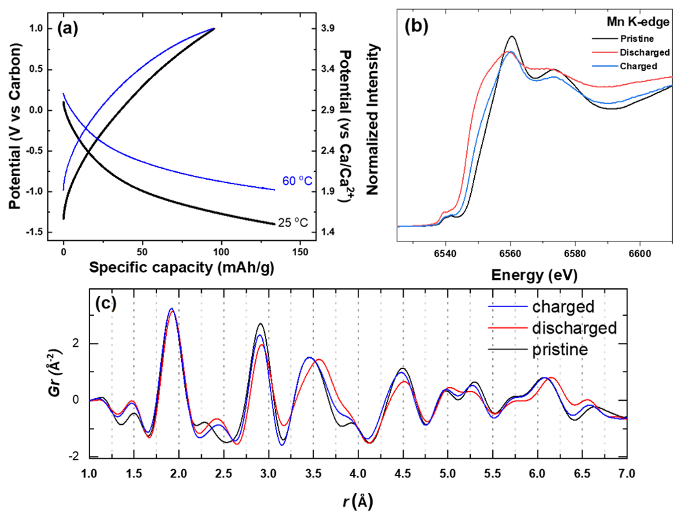
Nanocrystals of layered MnOx containing a high concentration of atomic defects and lattice water are shown to have remarkable electrochemical activity towards Ca2+ , amounting to a capacity of ~130 mAh/g at room temperature. Multimodal characterization revealed the notable degree of intercalation by probing the structural, compositional and redox changes undertaken by the defective MnOx nanocrystals.
Significance and Impact
Our results uncover a new candidate for a cathode in Ca-ion batteries operated by an intercalation mechanism at moderate temperatures, and cast light onto a structural family whose very high diversity makes it an attractive playground for further exploration.
Research Details
- The local structure of MnOx was found to contain a large concentration of interlayer Mn defects, creating clustering of walls that resembled the triple chains of todorokite.
- The observation of a virtually complete loss of activity upon dehydration and crystallization hints at the critical role of defects and lattice water within the nanocrystals in facilitating the migration of Ca2+.
DOI: 10.1021/acs.chemmater.1c03803

