Scientific Achievement
This work combines Density functional theory, quasi-harmonic approximation and experiments to explore the synthesizability of several marginally stable antiperovskites (APs) and overall, has obtained good agreement between experiments and computation.
Significance and Impact
This work improves understanding and gives guidance on the synthesis of alkali metal AP solid electrolytes with high ionic conductivity.
Research Details
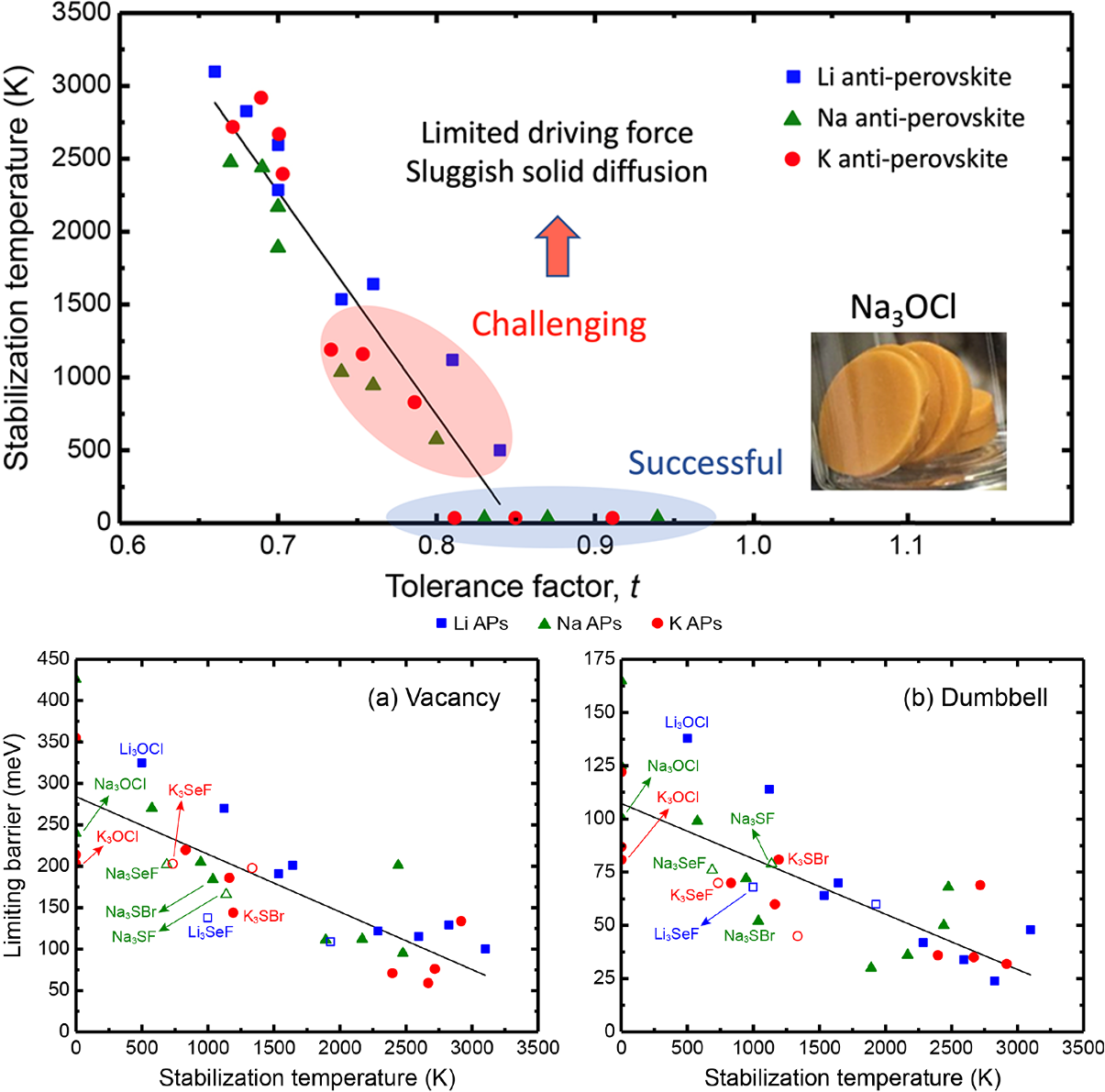
- Density functional theory calculations, in combination with the quasi-harmonic approximation, were used to predict the free energy change for synthesis reactions involving 36 alkali metal-based APs.
- A linear correlation is observed between the degree of lattice distortion and the stabilization temperature of APs, indicating that APs with the highest ionic mobility generally require the highest synthesis temperature.
- Computational results were used to guide experimental synthesis efforts of APs by estimating the temperatures above which a given AP is expected to be thermodynamically stable and the study has obtained good agreement between experiments and computation.

