Scientific Achievement
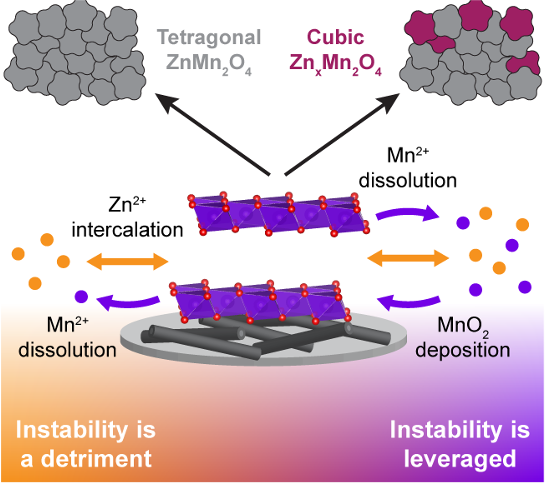
This report illustrates how dissolution, once seen as a fundamental drawback for MnO2 cathodes, can fortify capacity in AZIBs and provides guidance for expanding this concept and developing other systems that can reversibly deposit and dissolve the active material in a dynamic manner.
Significance and Impact
This work illuminates a path forward for unlocking the potential of batteries made with materials that are fundamentally unstable in their operating environment.
Research Details
- The dissolution of MnO2was revealed and employed as a mechanism for a functioning battery to replace the Zn-insertion mechanism.
- With clear focus on the MnO2dissolution/redeposition dynamics, it is evident that developing the ability to facilitate the electrochemical instability of MnO2will be critical to the performance of Zn/MnO2 batteries.

