Scientific Achievement
Typically, the solvent is assumed to be immobile in a polarized electrolyte. However, recent JCESR measurements show non-zero solvent velocity. This article proposes a continuum theory to predict such solvent motion and its influence on other electrolyte fields.
Significance and Impact
Often electrolyte transport properties are obtained by assuming that the solvent is immobile. We show this is an inaccurate assumption and provide a consistent framework to account for solvent motions across different experiments for measuring electrolyte properties. This work is critical to accurately screen electrolytes for new batteries.
Research Details
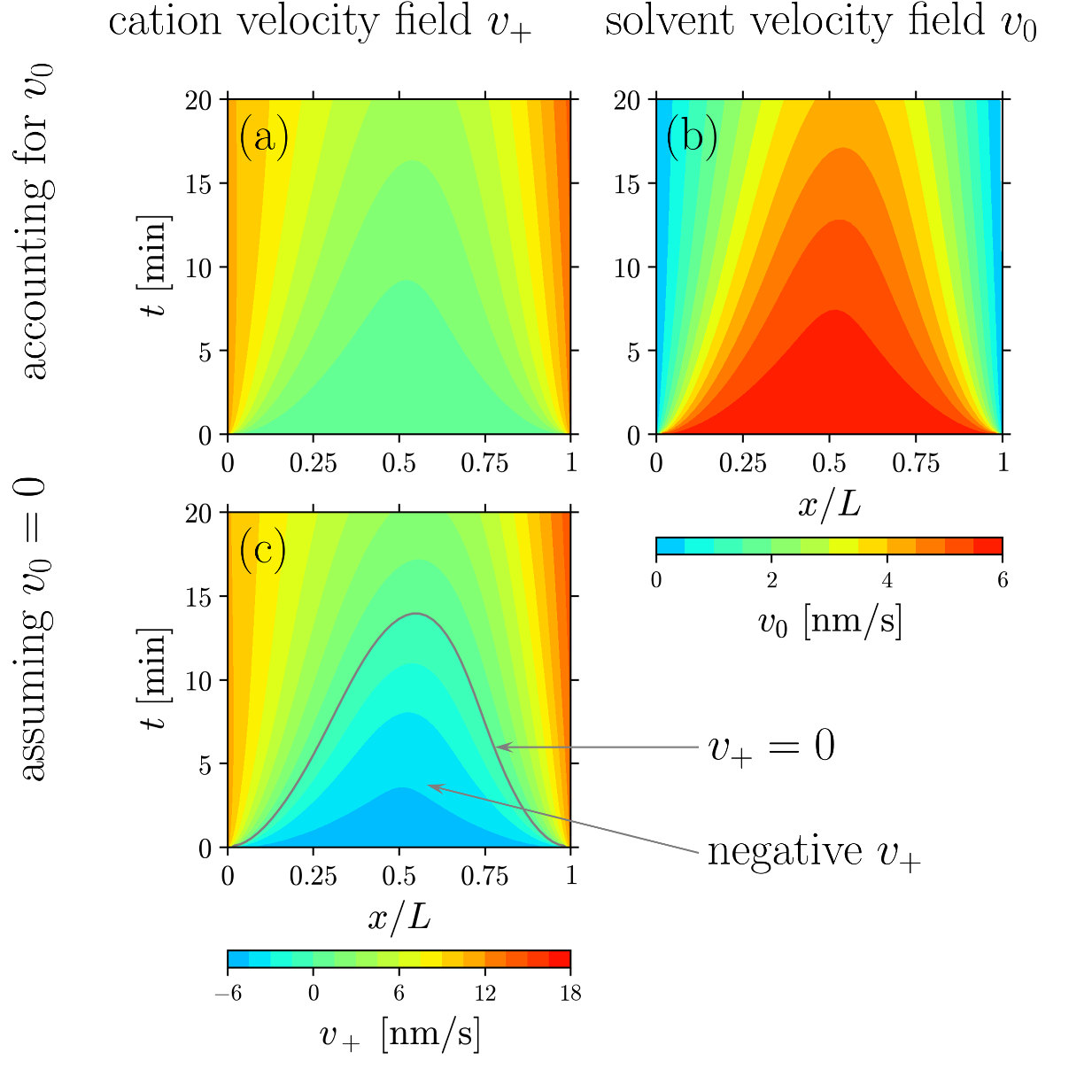
- LiTFSI/PEO electrolyte exhibits a negative cation transference number at 2.58 M salt concentration.
- If the solvent dynamics is not accounted for, one would expect negative cation velocity at short times as shown in (c).
- Instead, when accounted for the solvent dynamics ((a) and (b)), the cation velocity is always positive for this situation.
- Electrophoretic NMR (eNMR) is a technique for measuring transference number based on cation velocity at short times. Comparing (a) and (c), one can justify the importance of solvent motion in analyzing eNMR measurements.

