Scientific Achievement
The structural and chemical evolution of electrolyte constituents at the nanometric MgO surface were identified, providing a fundamental understanding of heterogeneous interphase evolution.
Significance and Impact
Revealing complex interactions of electrolytes with MgO surfaces, thus understanding the steps leading to the formation of complex interphases, provides insight into potential failure modes of Mg-batteries and enables pathways toward building directed interphase for optimal charge transfer processes.
Research Details
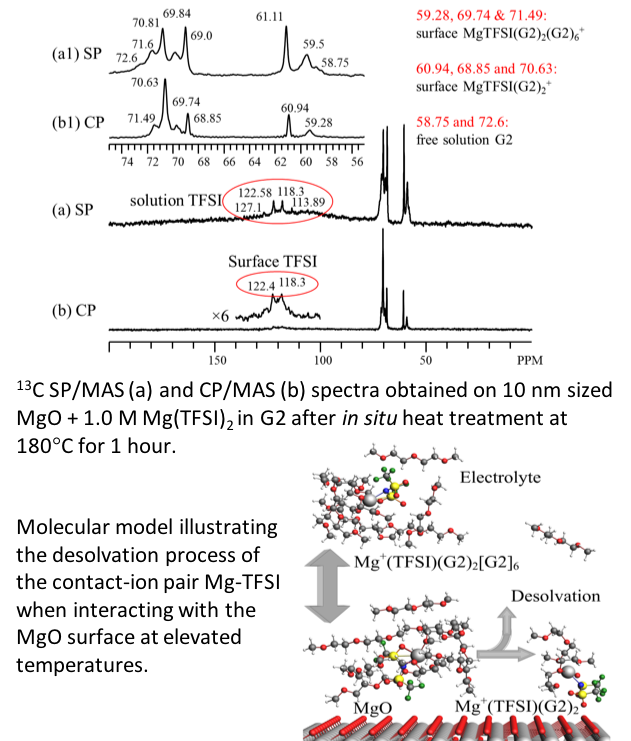
- At elevated temperatures, neat diglyme (G2) decomposes to methoxy species that adsorb onto the MgO passivation layer
- G2 represents the major MgO-adsorbed species for electrolyte solutions with 0.1 M Mg(TFSI)2 in G2.
- For 1.0 M Mg(TFSI)2 in G2, the major surface adsorbed species are G2-solvated Mg-TFSI contact ion pairs with the first shells containing 2 G2 molecules and the second shell containing either 0 or up to 6 G2
- A desolvation pathway for the Mg-TFSI contact ion pairs is evident on the MgO surface, revealing a correlation between surface adsorbed species and solvation structures in solution

