Scientific Achievement
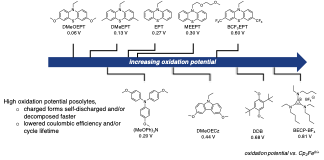
The charged forms of high oxidation potential posolytes exhibited either self-discharge or a combination of self-discharge and molecular degradation, limiting CE and/or cycle lifetime in the galvanostatic cycling, presenting a challenge of utilizing high potential compounds in nonaqueous RFBs.
Significance and Impact
Trace impurities present in the electrolyte system trigger radical cation decay at lower concentrations. Variation of the supporting electrolyte and solvent may provide a viable pathway for minimizing self-discharge and other decomposition pathways in nonaqueous RFBs.
Research Details
- The charged forms of posolytes were synthesized by chemical oxidation, and the oxidized products were isolated as solids for UV-vis stability studies.
- The variation of coulombic efficiency versus oxidation potential was examined using bulk electrolysis cycling at variable concentrations of selected active species and at variable charge/discharge rates.

