Scientific Achievement
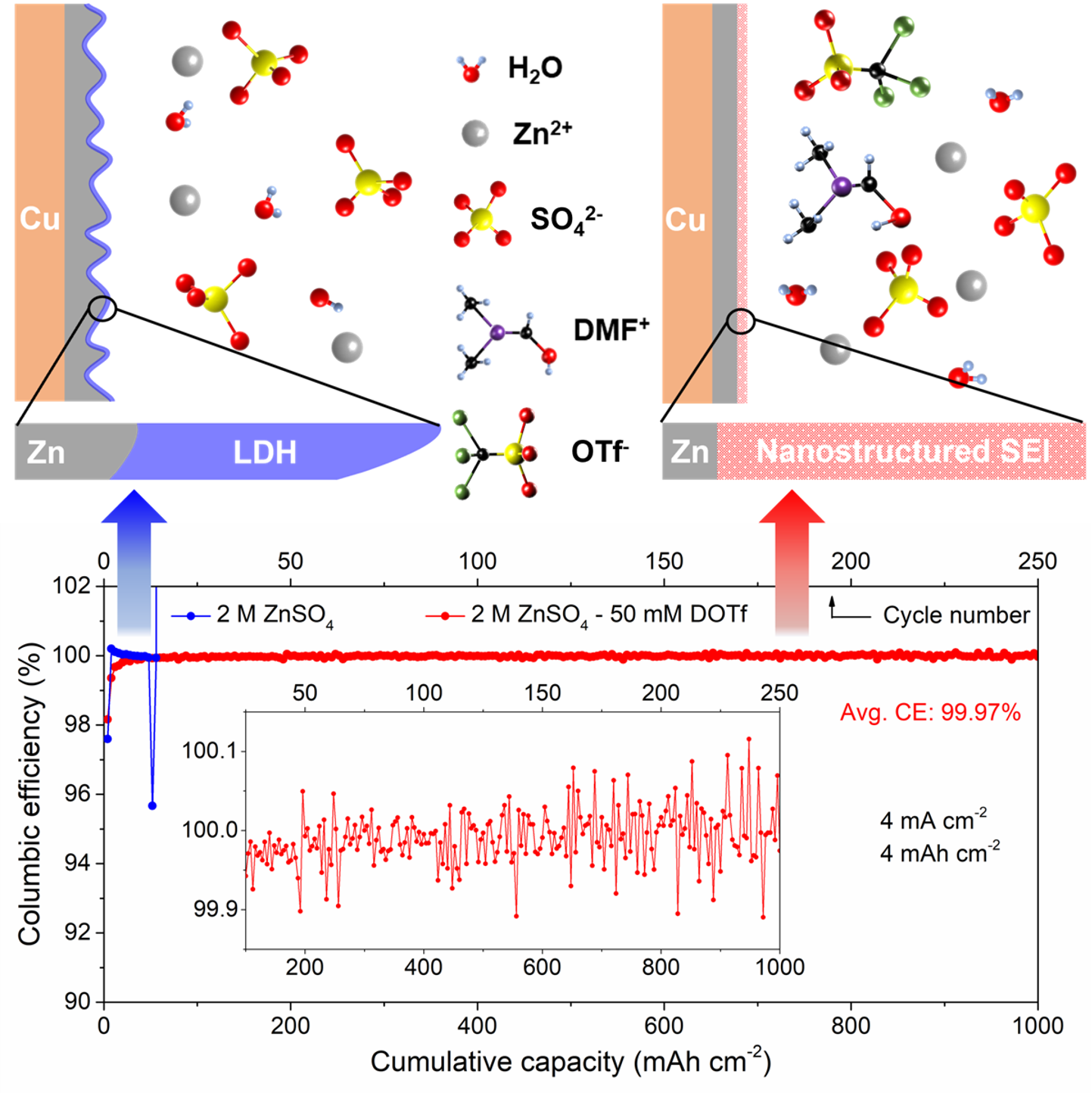
We introduced – N,N-dimethylformamidium trifluoromethanesulfonate (DOTf) – as a novel low-concentration electrolyte additive for aqueous Zn metal batteries. This electrolyte leads to dendrite-free and highly reversible Zn plating/stripping with close-to-100% average CE at practical cycling conditions (current density of 4 mA cm-2 and areal capacity of 4 mAh cm-2) with long cycle life.
Significance and Impact
We identified the origin of superior Zn stability and reversibility: an in-situ formed robust nanostructured SEI created by the water-assisted dissociation of DOTf into triflic superacid. This leads to the suppression of detrimental side-products and a highly modified nucleation-growth process on Zn electrodeposition at the nanoscale. Our findings and understanding of this novel electrolyte additive could potentially accelerate the commercialization of aqueous Zn metal batteries.
Research Details
- DOTf was introduced as a low-cost aqueous electrolyte additive to effectively suppress Zn dendrites and enhance Zn anode reversibility.
- Near 100% average CE for Zn plating/stripping was achieved under practical cycling conditions.
- An in-situ formed robust, water-excluding, complex nanostructured SEI was revealed by operando spectroscopy (UIUC ) and imaged by cryo-microscopy (SNL).
- The modified electrolyte enables long-term stable cycling of Zn||Zn0.25V2O5×nH2O full cells at low N/P ratios and practical current densities and areal capacities suitable for mini-grid storage.

