Scientific Achievement
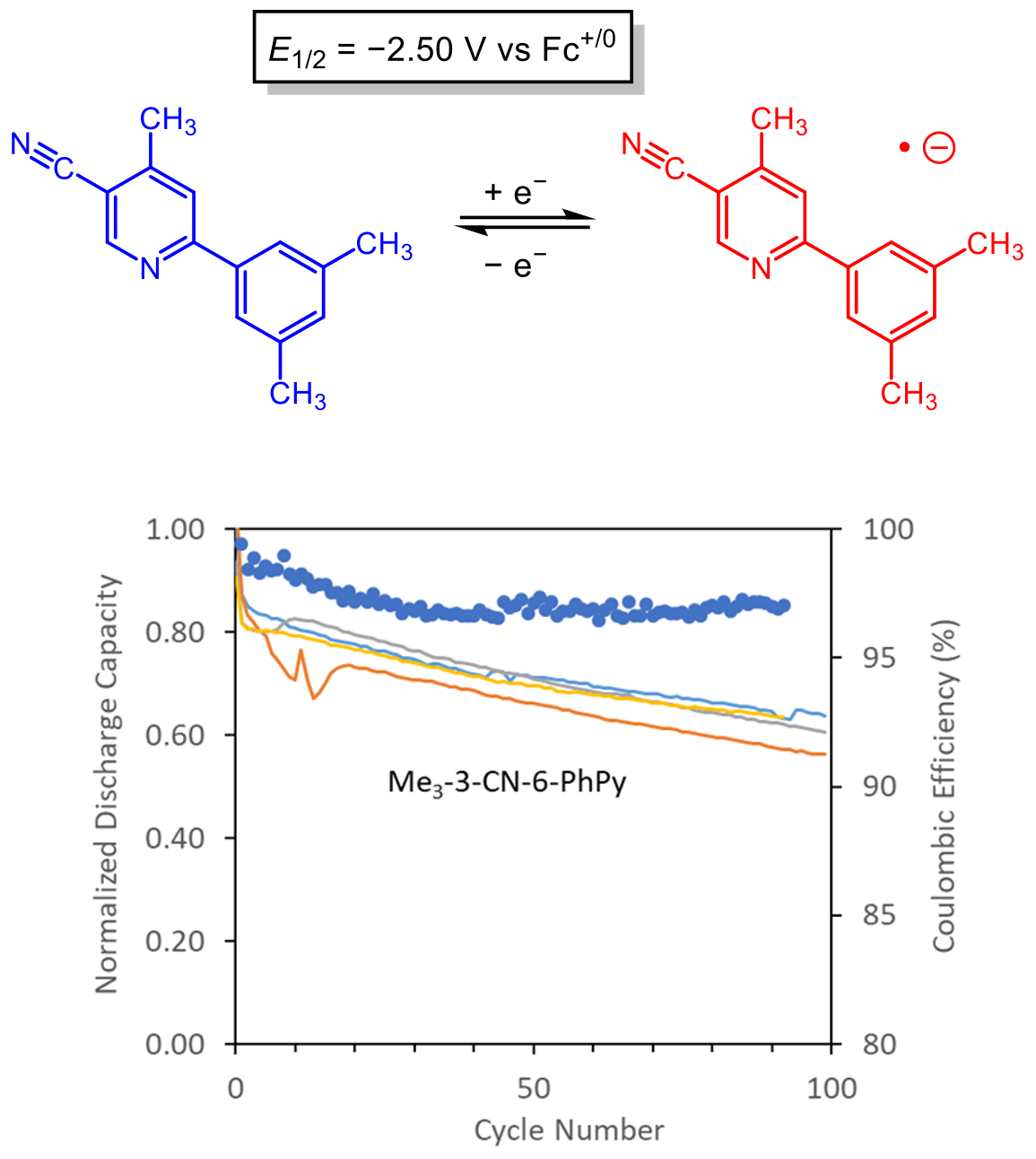
Discovery of a cyanophenylpyridine derivative with a very low reduction potential and good stability during cycling.
Significance and Impact
Nonaqueous solvents have a much larger electrochemical window than aqueous systems, and practically viable nonaqueous redox flow batteries will require redox-active molecules that utilize that window, as this optimized cyanophenylpyridine derivative does.
Research Details
- The three cyanopyridine isomers and all 10 cyanophenylpyridine isomers were examined computationally and experimentally, where reduction potentials, propensity for dimerization of the radical anion, and cycling stability were determined.
- The 3-cyano-6-phenylpyridine isomer with three strategically placed methyl groups had a very low reduction potential, good cycling stability, and a radical anion with a little propensity for dimerization.

