Scientific Achievement
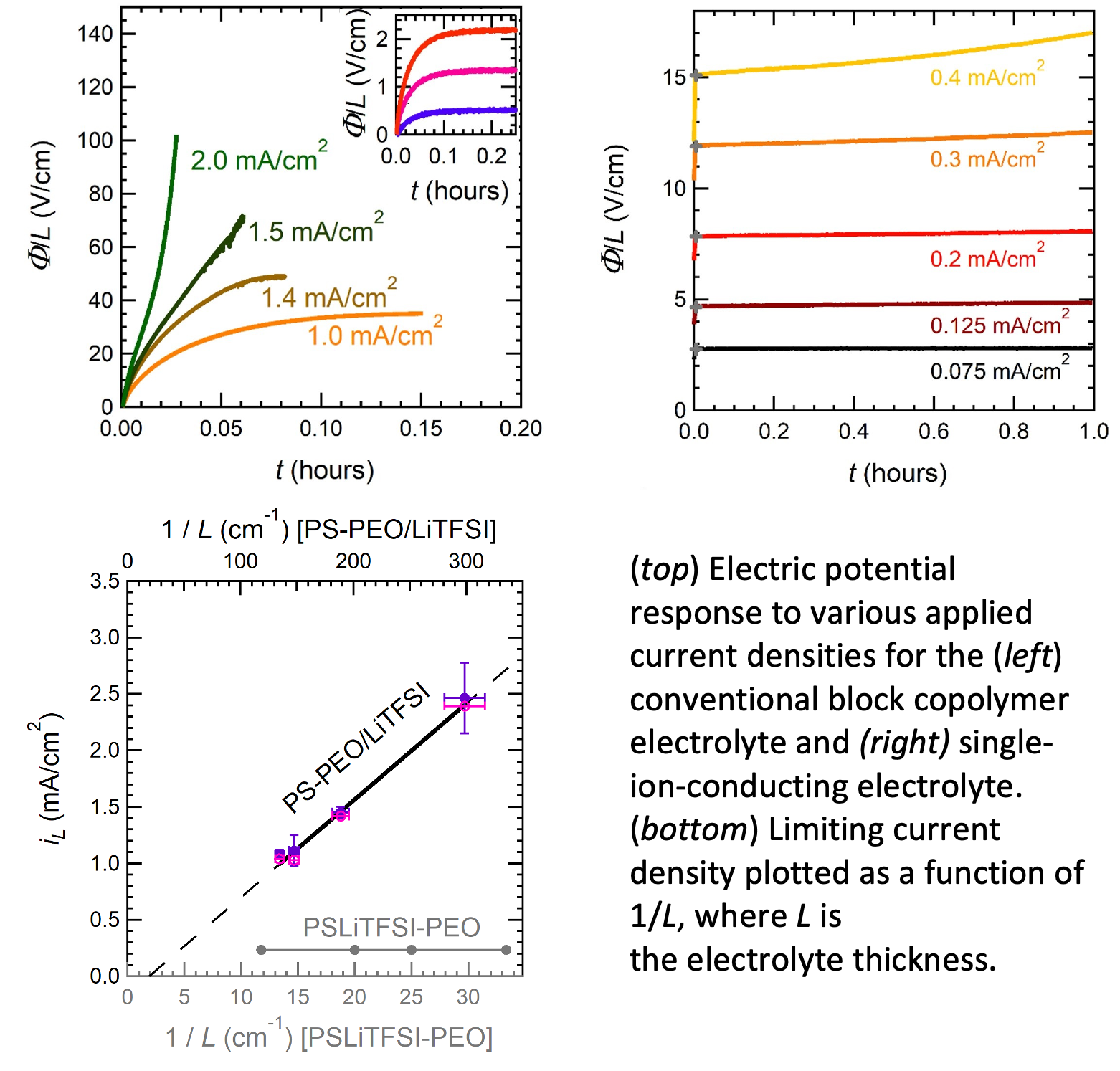
The performance of an electrolyte in a battery is determined by the limiting current – the maximum allowable current before detrimental irreversible side-reactions take over. We have developed a methodology for determining limiting current in electrolytes with mobile cations and anions (conventional liquid electrolytes) and electrolytes with only one mobile ion (single-ion conductors such as inorganic ceramics and glasses).
Significance and Impact
The limiting current of the single-ion-conducting electrolyte was found to be independent of electrolyte thickness, contrary to conventional electrolytes where limiting current is inversely proportional to thickness. This result demonstrates a lack of ion concentration gradients within single-ion-conducting electrolytes.
Research Details
- The fitted slopes of electric potential responses were observed as a function of current densities to determine limiting current.
- This new method for determining limiting current is applicable to a wider range of electrolytes.

