Scientific Achievement
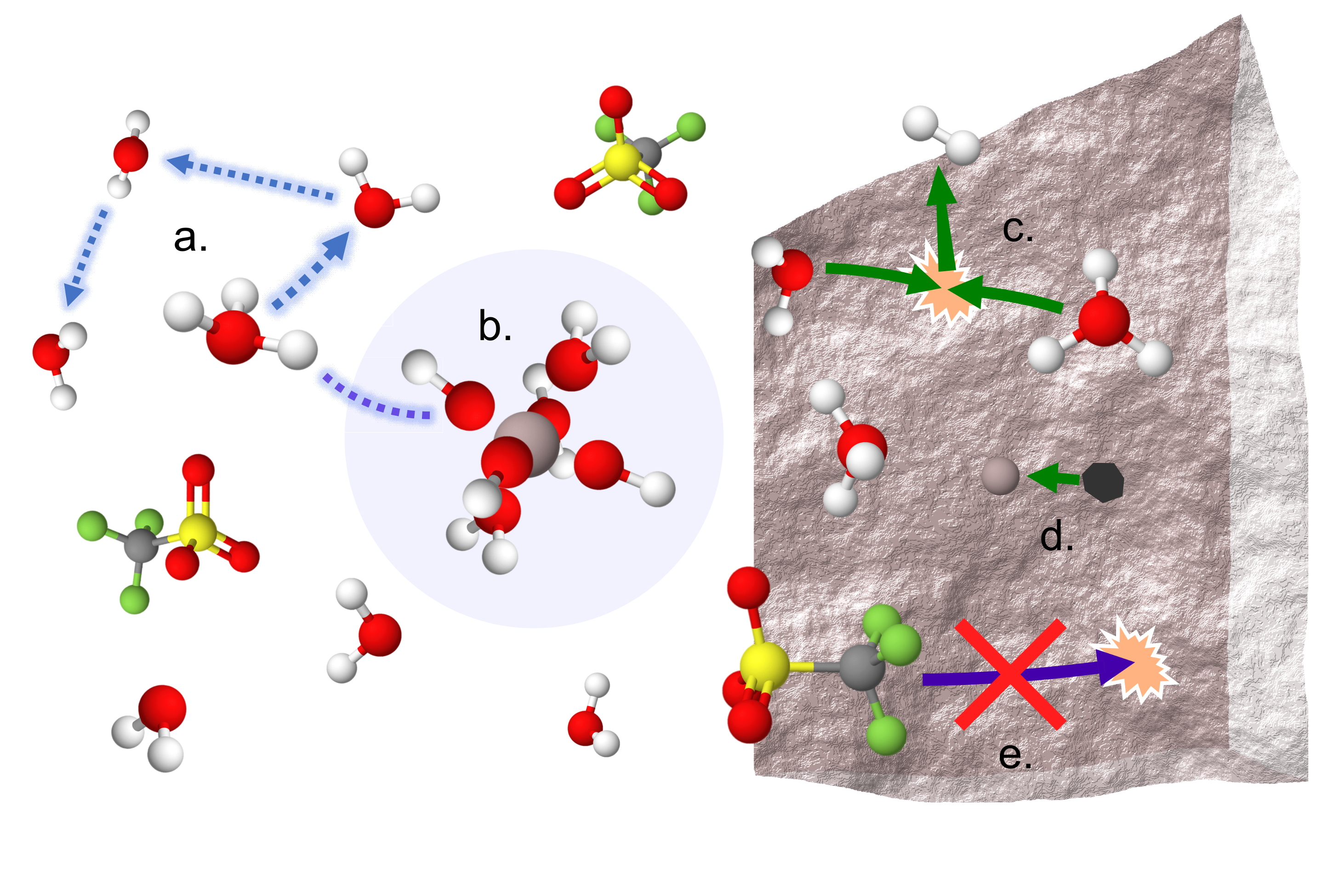
We revealed the first compelling evidence for a dynamic octahedral solvation structure around Al3+ dominated by labile water and OH-, without Al-OTf contact ion pairs, at high salt concentrations. High proton activity is observed in transport and electrochemical measurements which relates well with the proposed solvation environment.
Impact
We also exposed practical concerns related to (i) the corrosiveness of the acidic aqueous solutions, (ii) the degree of hydration of Al(OTf)3 salt, and (iii) the grossly insufficient reductive stability (>1 V between HER onset and Al3+/Al). Collectively, these factors constitute multiple fundamental barriers to the feasibility of rechargeable aqueous Al batteries.
Experimentalists:
- Glenn Pastel (ARL): Electrochemistry/Physicochemical
- Ying Chen (PNNL): Liquid & solid-state NMR
- Travis Pollard (ARL): Born-Oppenheimer molecular dynamics simulations
- Allen Zheng (Hunter College): PFG-NMR (diffusivity)
- Nathan Hahn (Sandia): Dielectric Relaxation Spectroscopy

