Scientific Achievement
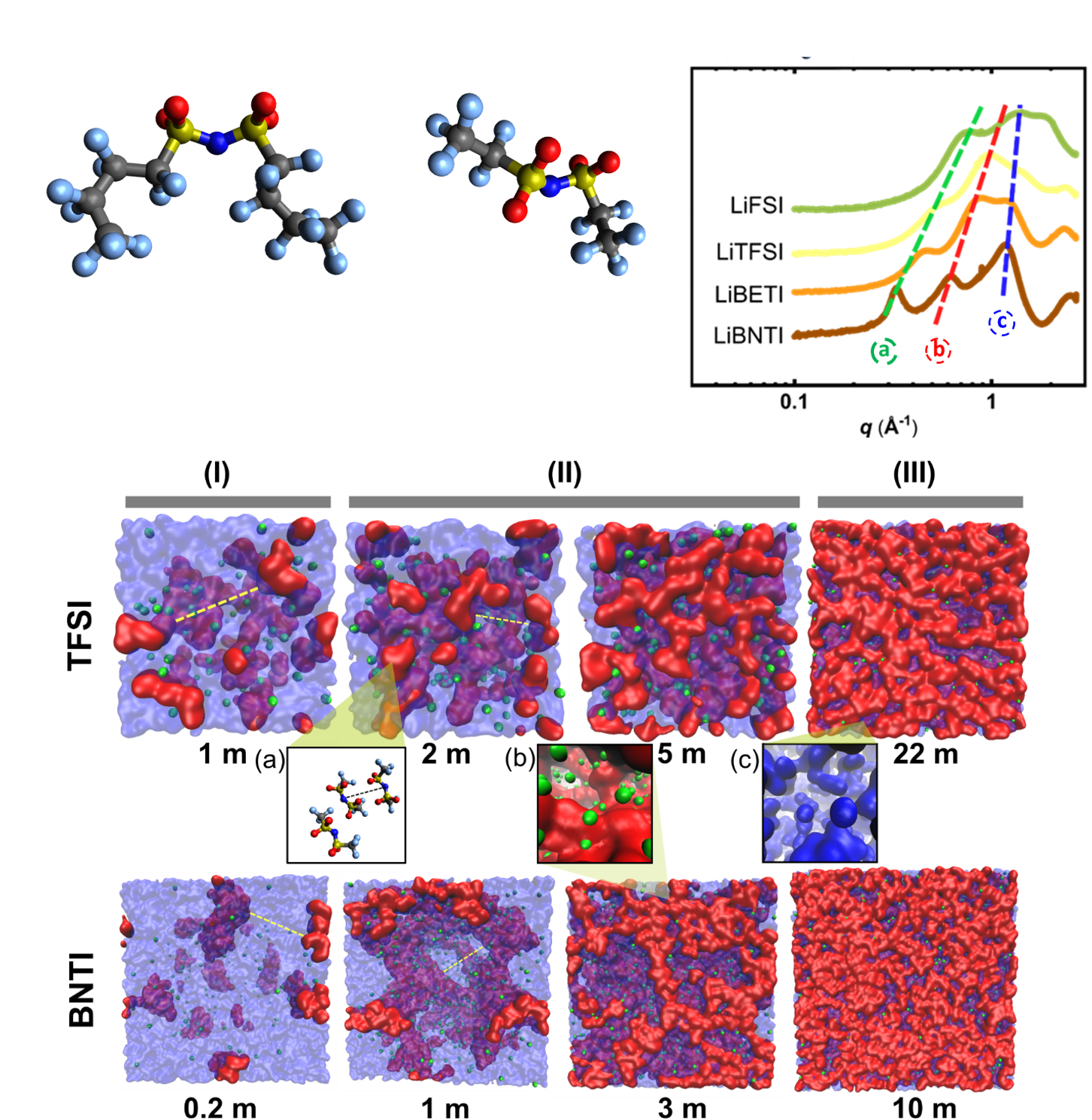
We studied the structures of a series of imide-based lithium aqueous solutions: bis(fluoro sulfonyl)imide (FSI), bis(trifluoromethane sulfonyl)imide (TFSI), bis(pentafluoroethane sulfonyl) imide (BETI), and bis(nonafluorobutane sulfonyl)imide (BNTI) at various concentrations through small angle X-ray scattering/wide angle X-ray scattering (SAXS/WAXS) and molecular dynamics (MD) simulations
Significance and Impact
We found two competing structures, anion solvated structures and anion networks, in the four aqueous solutions throughout three concentration regimes: low, medium, and high. The high concentration regime has only anion network structures which might explain the high electrochemical stability window. We also observed that the transition from one structure to the other is predominantly controlled by the salt volume fraction for all four imide-based lithium salts aqueous solutions.
Research Detail
- SAXS/WAXS results show that two competing structures existed in the four lithium salts aqueous solutions: anion solvated structure and anion network.
- The anion network plays a crucial role in obtaining a stable and enlarged electrochemical window.
- MD simulation results reveal that the formation of anion networks starts at around 20 % of the salt volume fraction for all imide-based lithium salts aqueous solutions.
- The d spacing of the anion solvated structure follows a linear correlation with the number of carbons in the fluorocarbon chains and an exponential correlation with the concentrations.

