Scientific Achievement
The speciation trends of weakly-coordinating multivalent salts in ethereal solvents were established, providing a generalized understanding of how multivalent ion correlations impact electrolyte transport and stability.
Significance and Impact
Understanding ion correlations in ostensibly “fully dissociated” electrolytes establishes realistic limits of ion de-correlation in solvents suitable for multivalent ion batteries. This investigation further identified the non-intuitive design rule that higher salt concentrations lessen ion correlations and attendant stability issues in such solvents.
Research Details
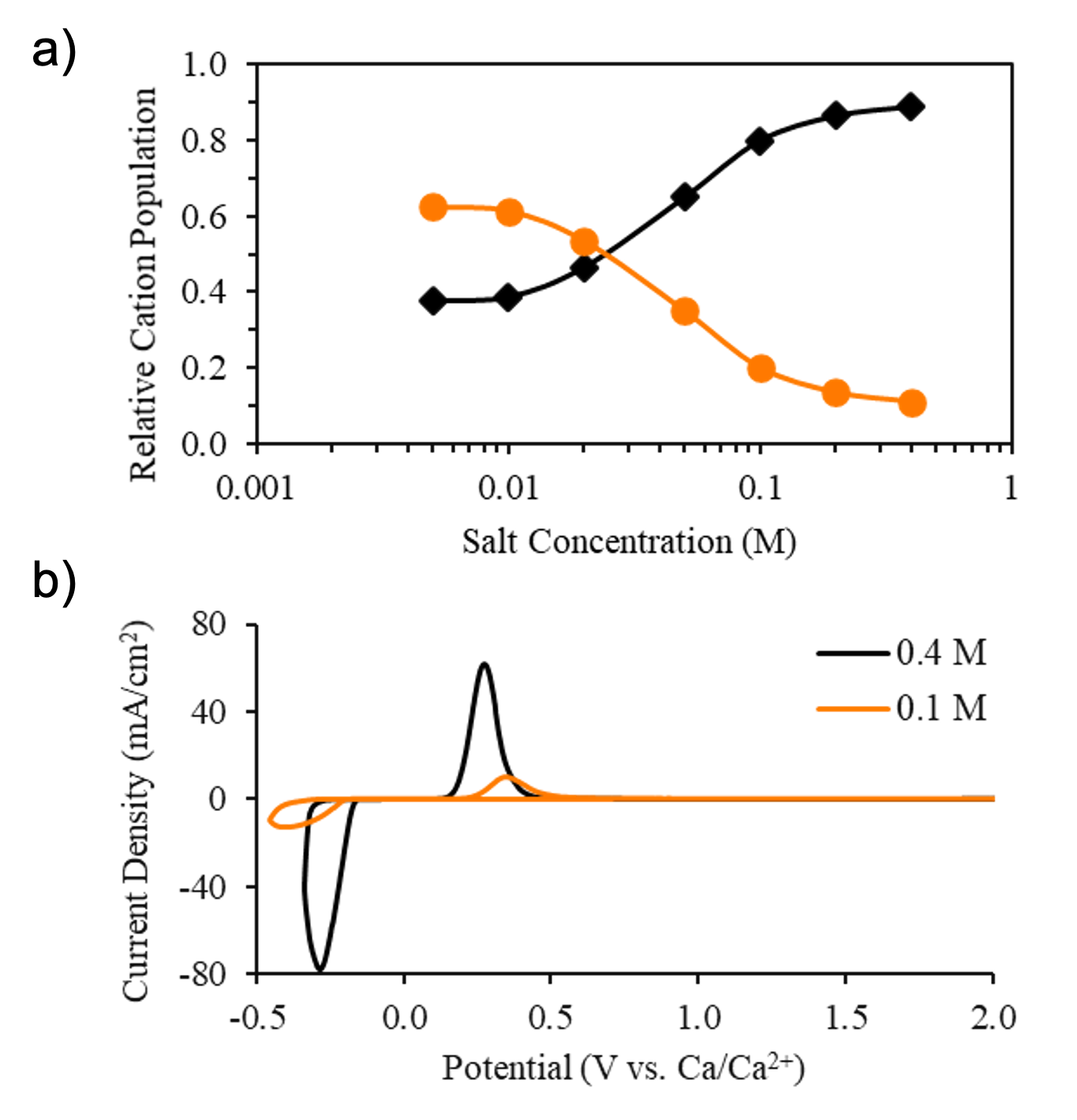
The ionic conductivity and diffusivity trends of calcium tetrakis(hexafluoroisopropoxy)borate (Ca(BHFIP)2) dissolved in THF or DME indicate that non-intuitive concentration-dependent ion correlations are present.
Coupled dielectric relaxation spectroscopy and MD simulation indicate that these correlations involve an equilibrium between free ions and solvent-separated ion pairs (SSIPs).
X-ray absorption spectroscopy, combined with time-dependent DFT calculations, confirm concentration-dependent changes in solvation structure consistent with free Ca2+ vs. SSIP Ca(BHFIP)+.
High concentration regimes maximize free Ca2+ fraction and minimize BHFIP- anion decomposition, leading to improved calcium metal cycling efficiency.

