Scientific Achievement
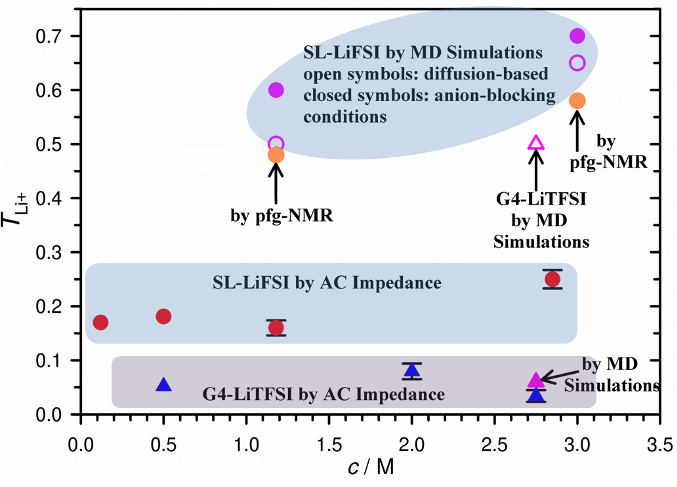
A systematic study of the effect of concentration on Li+ transference number under anion-blocking conditions (T_(〖Li〗^+)^abc) in sulfolane (SL) and tetraglyme (G4) using VLF-IS showed that SL is a more superior solvent than G4 for Li+ transport, even though the viscosity of the former is higher but with similar ionic conductivity.
Significance and Impact
Cation-solvent interaction strongly affects cation transport in concentrated systems—the faster Li+ transport in the SL systems is a result of the ion-hopping diffusion mechanism facilitated by the long-range liquid structures of SL molecules and the anions, while the sluggish Li+ transport in the G4 systems is dominated by the vehicular-typed diffusion mechanism resulted from the formation of stable [Li(G4)]+ solvation cages.
Research Details
- VLF-IS was employed instead of pfg-NMR because the latter assumes no interactions between ions and solvent molecules which is not applicable to concentrated systems.
- This technique together with the special sample holder with adjustable electrode distance allows salt-diffusion coefficient determination.
- Frequency from 1 MHz to 0.1 mHz, where the low range captures the diffusion impedance, at 2 mVrms to avoid convective effects.
- T_(〖Li〗^+)^abc=R_bulk∕(R_bulk+R_diffusion )

