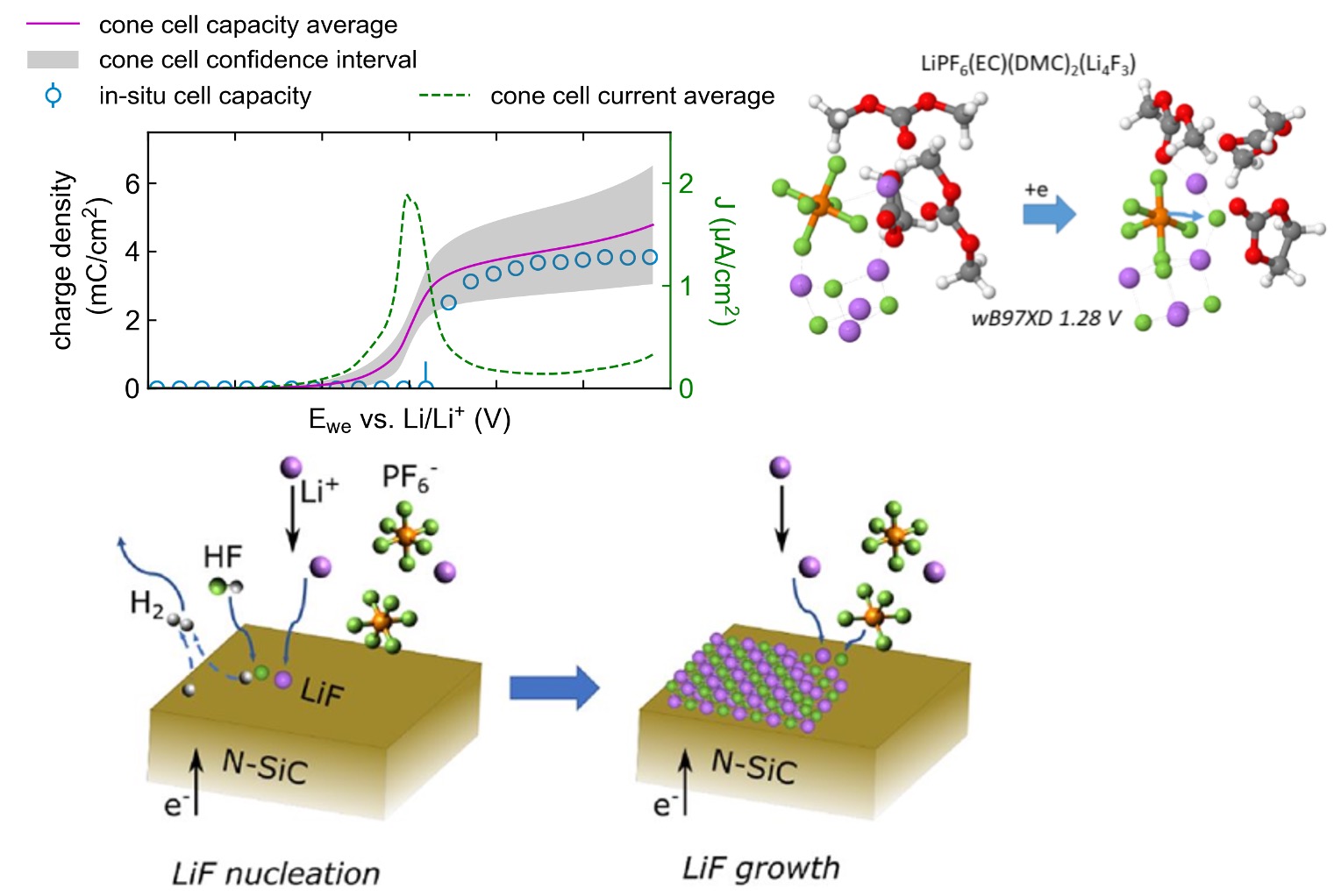
Scientific Achievement
We revealed the origin of LiF in the solid electrolyte interphase (SEI) using a multimodal experimental and theoretical approach and found that LiF nucleated via the electrocatalytic transformation of HF followed by significant direct anion reduction.
Significance and Impact
Our results provide significant novel insight into how interrelated processes play a role in surface electrochemical reactions, and the findings are essential to understanding the formation mechanism and properties of SEI layers.
Research Details
- Precision electrochemistry in cone cell with N-SiC model electrode, which is electrochemically non-active and conducting, allowing for straightforward interpretation of electrochemical and structural measurements
- Ex-situ compositional characterization with XPS suggests the SEI mainly consists of LiF.
- Operando X-ray reflectivity (XRR) tracks the structural evolution of the SEI film.
- Operando X-ray diffraction (XRD) monitors the structure and texture evolution of LiF during the first two CV cycles.
- Quantum Chemistry Calculations and Born-Oppenheimer Molecular Dynamics simulations indicates that LiF nucleates via HF splitting, then direct anion reduction dominates.

