Scientific Achievement
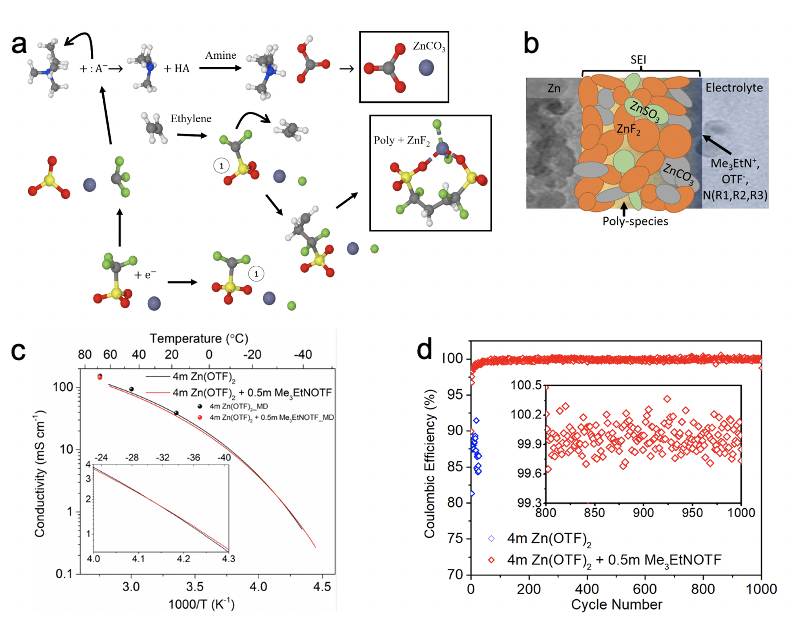
A novel, dilute dual salt electrolyte promotes formation of a robust and hydrophobic fluorine-rich interphase that suppresses the parasitic hydrogen evolution reaction, enabling highly reversible and dendrite-free Zn plating/stripping and supporting a 325 Wh kg-1 Zn-air full cell for 300 cycles. The merits of this electrolyte are further demonstrated under challenging limited Zn conditions with multiple intercalation cathodes.
Significance and Impact
The use of a dilute electrolyte overcomes cost and conductivity issues associated with superconcentrated electrolytes, while the small amount of trimethylethyl ammonium triflate shows an oversized effect on interfacial structure (water exclusion) and interphase formation. These results will motivate continued exploration of novel supporting salts and/or additives to address Zn irreversibility in aqueous electrolytes.
Research Details
- Fluorinated interphase from anion decomposition was characterized by spectroscopic methods and DFT.
- The alkylammonium cation was predicted to decompose to ethylene (radical scavenger) and trimethylamine.
- Synergistic reactions between dissolved gasses and amines were predicted as a source of carbonates in interphase.
- Polarizable force fields accurately predict ionic transport, bulk solvation structure, and double layer composition.

