Scientific Achievement
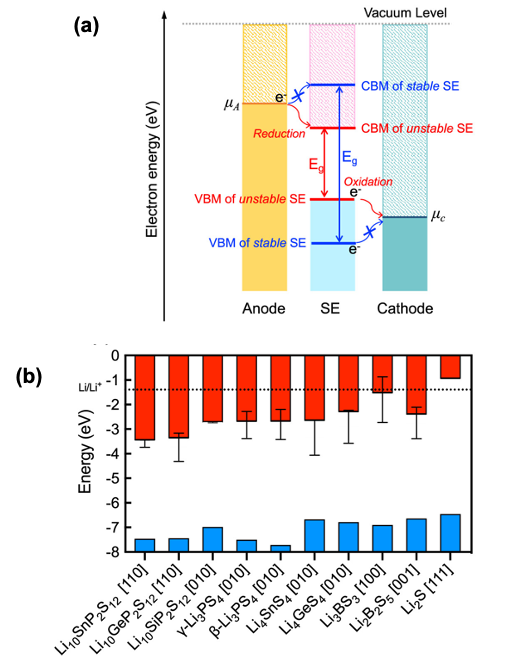
The likelihood for charge injection from a Li metal anode to 10 sulfide-based SEs was determined by computing the positions of the SE’s band edges with respect to the electrochemical potential of the electrode. Trends in charge transfer stability were compared to those for chemical stability with a Li anode and were found to be similar. The combined characterization of chemical and charge transfer phenomena allows for a comprehensive assessment of interfacial stability.
Significance and Impact
Reduction or oxidation of a solid electrolyte (SE) by the electrodes of a battery can inject electrons or holes into the SE, inducing unwanted electrical conductivity and/or precipitating harmful interfacial reactions. The present study demonstrates a methodology for predicting these degradation modes.
Research Details
- Bandgaps and the absolute positions of band edges of SEs were evaluated using many-body perturbation theory.
- Although all SEs examined exhibit large band gaps (>4 eV), nearly all are susceptible to electron injection.
- The greatest resistance to electron injection was exhibited by Li3BS3, suggesting that it may be a promising SE
- The utility of the present approach is further demonstrated by employing it to interpret recent experiments on the Li/Li2H2PO4/LGPS interface system.

