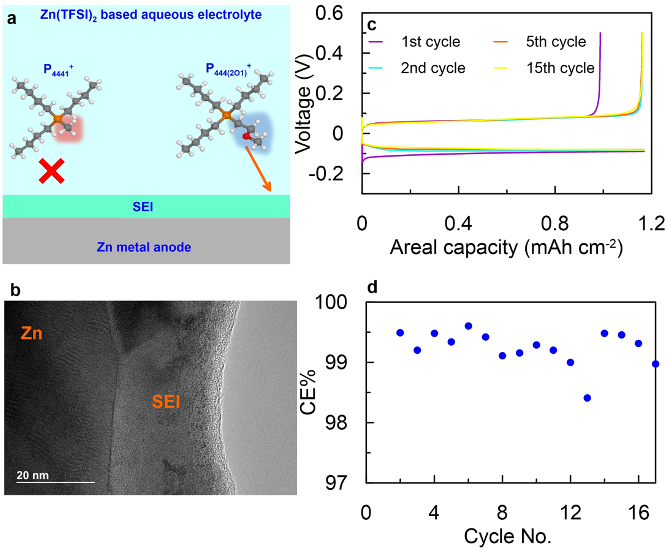
Scientific Achievement
The presence of P444(2O1)+ cation in aqueous electrolyte enhances Zn reversibility dramatically by enabling a high Coulombic efficiency (CE, > 99%) at high utilization (20% Zn per cycle) for Zn plating/stripping, suppressing hydrogen evolution, and allowing a remarkable dendrite-free cycling even under aggressive conditions (2.5 mA/cm2, 2.5 mAh/cm2).
Significance and Impact
We correlate the enhanced Zn reversibility with interfacial structure and interphasial chemistries modification. This presents a promising new direction to address the irreversible issues of a Zn metal anode in mild acidic aqueous electrolytes with the addition of reactive supporting cations
Research Details
- The effect of these supporting salts on bulk electrolyte structure was also investigated using small angle neutron scattering (SANS), infrared spectroscopy (FTIR), and Raman spectroscopy.
- Combining sputtering XPS, TEM and DFT calculation, we revealed the different electrochemical behaviors and interphasial chemistries induced by the chemical functionalities on phosphonium cations P4441+ and P444(2O1)+

