Scientific Achievement
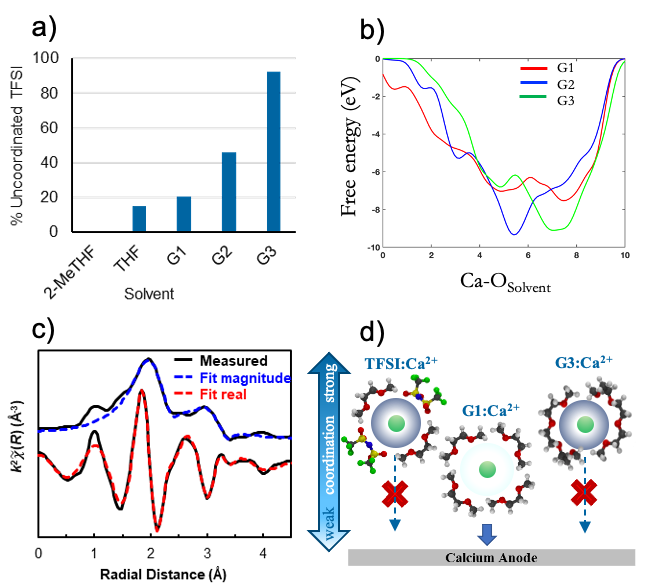
Ethereal solvent structure determines Ca2+ solvation structure and the efficiency of calcium metal plating. In a moderate-coordinating solvent such as G1, anion coordination determines whether or not reversible plating takes place while in a strongly coordinating solvent such as G3, calcium plating is frustrated by a rigid solvation shell, regardless of anion.
Significance and Impact
These findings identify specific stability challenges in weakly coordinating and wide electrochemical window MV electrolytes while laying the groundwork for understanding and controlling coordination-stability relationships therein.
Research Details
Raman spectroscopy reveals the relative coordination strengths of typical ethereal multivalent battery solvents.
AIMD/metadynamics simulations rationalize these fundamental coordination relationships through free energy landscapes in Ca2+-TFSI-glyme electrolytes.
X-ray absorption spectroscopy resolves the solvation structures present in ethereal CaTFSI2 and CaBHFIP2 electrolytes.
Electrochemical analysis connects these coordination tendencies directly to calcium electrodeposition behavior.

