Scientific Achievement
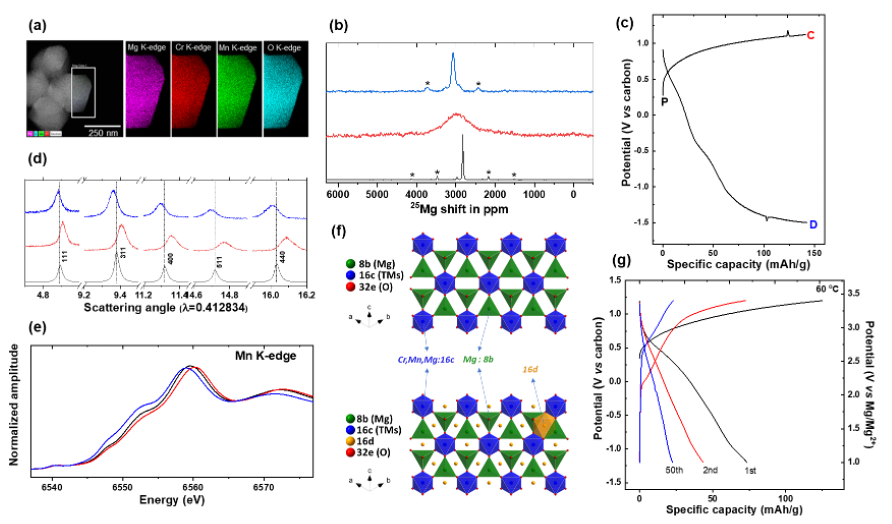
The capability of the tailored MgCrMnO4 spinel to (de)intercalate Mg2+ electrochemically at high potentials was evaluated by the theoretical and experimental approaches. High Mg2+ activity was observed in bulk, with a remarkable degree of lattice breathing and reversibility.
Significance and Impact
Lattice Mg2+ in a tailored solid solution spinel, MgCrMnO4, is electrochemically utilized at high Mn-redox potentials in a non-aqueous electrolyte. Possible cyclability at moderate temperature (60 ℃) was observed along with a ~180 Wh/Kg of energy density delivered at the first discharge reaction. This study redefines lattice design via chemical and structural composition of functional spinel oxides utilized by Mg2+ intercalation at high potentials. Our findings uncover a new subclass of cathode material for rechargeable Mg-ion batteries.
Research Details
The intrinsic design weaknesses in a single B site Cr- or Mn-spinel for a Mg-ion cathode are alleviated by building a solid-solution MgCrMnO4 where the mixed transition metal lattice provides mobility via Mg2+ bound by Cr3+ and suitable high redox potential via Mg2+ bound by Mn3+ for (de)intercalation.

