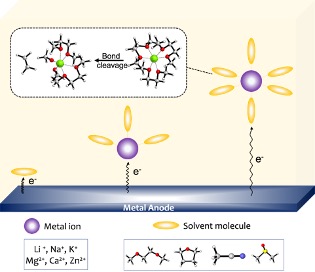
Scientific Achievement
Simulated molecular structures and the reductive stabilities of many monovalent and divalent metal ion complexes with organic solvents.
Significance and Impact
The data serve as a library of fundamental knowledge to enable deeper understanding of electrode-electrolyte interfacial structures and electrochemical reactions.
Research Details
- Performed density functional theory calculations of complexation structures and the electron affinities of 300 Metal (Li/Na/K/Mg/Ca/Zn)-solvent species.
- Identified linear relationships between binding enthalpies of Li+ ion and other monovalent and divalent metal ions with solvent molecules. Linear relationships identified between the binding energies of metal ions (M: Na+, K+, Mg2+, Ca2+, Zn2+) with dimethoxy ethane, tetrahydrofuran and H2O molecule(s) and corresponding lithium ion complexes.
- Simulations predict spontaneous reductive decomposition of dimethoxy ethane (DME) solvent in the presence of Mg2+ ion in the Mg2+-(DME)3 complex.

