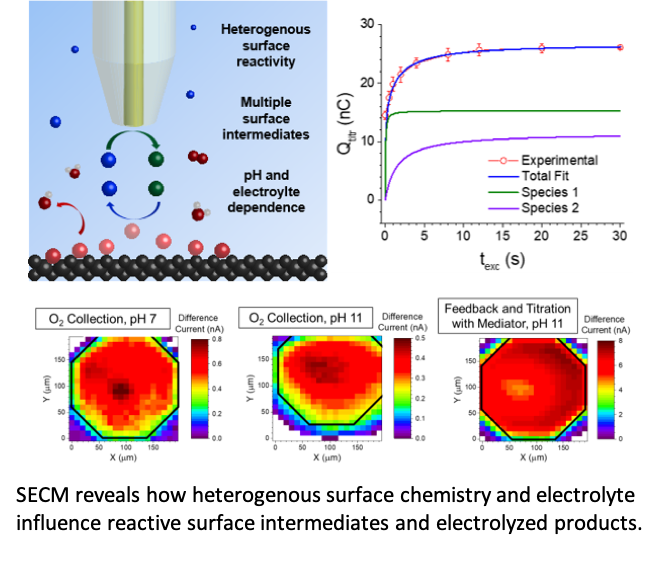
Scientific Achievement
Multiple surface oxygen species form and react at different rates on sp2 carbon-containing boron-doped diamond electrodes during water oxidation.
Significance and Impact
A new experimental approach allows dynamic probing of reactive species on electrode surfaces and easy discrimination between multiple surface species.
Research Details
- Scanning electrochemical microscopy (SECM) was used to quantify the surface coverage of surface-bound oxygen species during water oxidation. A new experimental protocol and theory quantitatively describe how these species evolve over time.
- The surface coverage and formation rates of these two intermediates depends on pH and electrode potential. The reactive oxygen species that are evolved from the surface are also strongly pH-, potential-, surface chemistry-, and electrolyte-dependent.

