Scientific Achievement
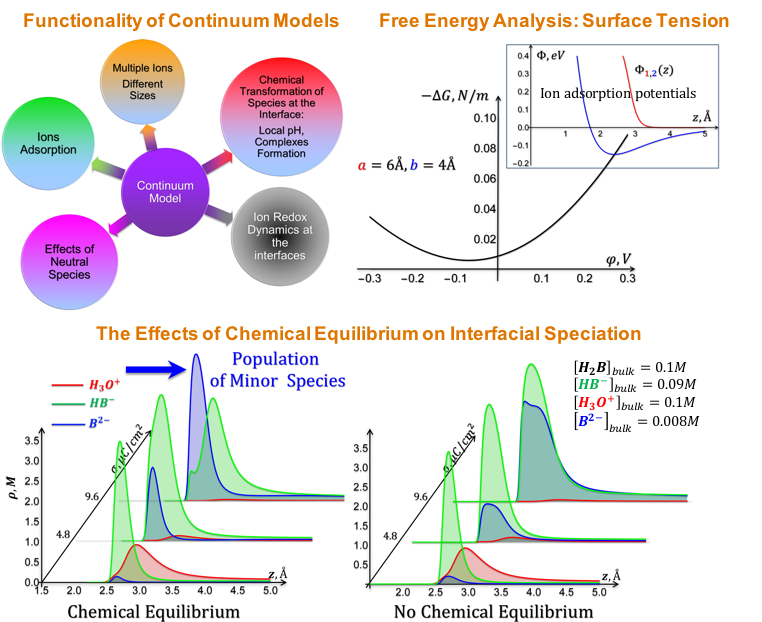
Development of a novel formalism and methodology to model electrolyte composition at electrified (electrode) interfaces. Predictions that minor species (from the bulk perspective) may become dominant at the electrified interface.
Significance and Impact
We have determined that Faradaic and Capacitive regimes of electrified interfaces may easily become computationally intractable using ab initio methods or that naïve calculations might unintentionally model unrealistic, nonequilibrium scenarios. Our models serve as a guiding component of a multiscale predictive methodology that retains atomistic details without compromising basic principles of thermodynamics.
Research Details
- Includes the description of electrolytes with multiple components, the effects of specific non-electrostatic interactions (specific adsorption), ion size disparity, and the explicit presence of neutral species (e.g. the solvent).
- Includes bias-dependent effects of local chemical transformations driven by equilibrium constants and/or electrochemical reactions
- Enables a description of (solid/gas)-liquid interfaces and predicts experimentally measurable characteristics (e.g., surface tension, adsorption isotherms, diff’l capacitance, etc.)

