Scientific Achievement
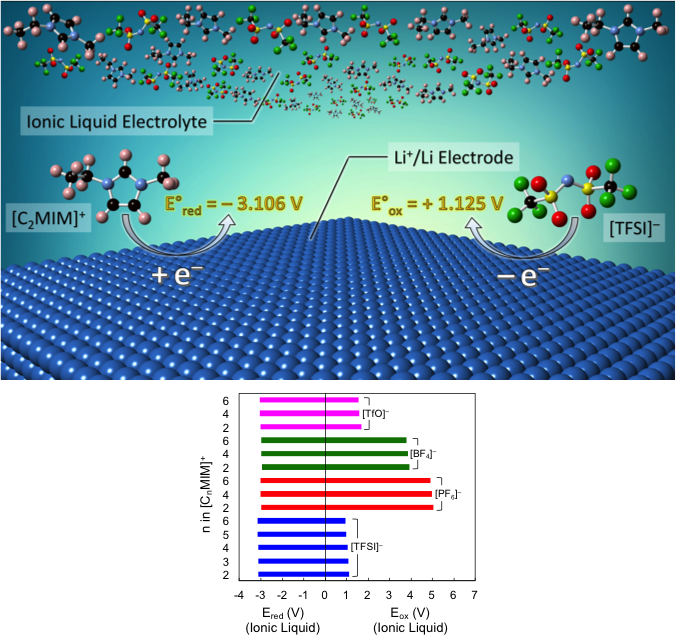
The oxidation and reduction potentials of the constituent cations and anions of a series of imidazolium-based ionic liquids with respect to a Li+/Li reference electrode were calculated using density functional theory (DFT) following the method of thermodynamic cycles.
Significance and Impact
Thermodynamic cycle method can be utilized to efficiently populate the electrochemical stability window (ESW) data within the ionic liquid database, which is used to guide the selection of ionic liquid electrolytes in designing high voltage rechargeable batteries.
Research Details
- DFT results show that the ESW of these ionic liquids is limited by the oxidation potential of anion and reduction potential of cation, when assuming single-electron transfer redox reactions.
- We found that [CnMIM]+[PF6]– are the most electrochemically stable ionic liquids due to the high oxidation potential of [PF6]– while [CnMIM]+[TFSI]– are the least stable ones among the ionic liquids examined in this work.
- Our calculations indicate that the ESW of these ionic liquids are insensitive to the length of alkyl side chain of the imidazolium cations.
Work performed at the University of Michigan (JCESR partner), Argonne National Laboratory (JCESR managing partner) and Washington State University by S. Kazemiabnavi, Z. Zhang, and K. Thornton, S. Banerjee. J. Phys. Chem. B, 2016, 120 (25), 5691–5702

