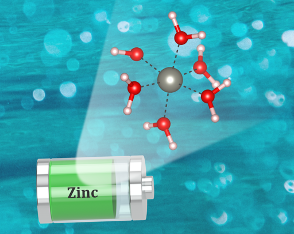
Scientific Achievement
Zn2+ cations are mainly solvated by six waters in their first solvation shell, while TFSI- anions are excluded from solvation in the WISE electrolyte consisting of 1 m Zn(TFSI)2 and 20 m LiTFSI although the TFSI- concentration is as high as 22 m.
Significance and Impact
The results shown here suggest that the unusual high stability of the Zn2+/Li+ WISE electrolytes is not due to the changes in the Zn2+ solvation environment in the bulk and additional work needs to be performed to explain the underlying mechanisms.
Research Details
▪Mixed Zn(TFSI)2/LiTFSI WISE electrolytes were studied using multimodal X-ray total scattering, X-ray absorption spectroscopy (XANES & EXAFS), FTIR experiments, and classical molecular dynamics (MD) simulations.
▪MD simulations were validated against X-ray total scattering and FTIR experiments.
▪EXAFS results were fit to model clusters that satisfy the symmetric octahedral coordination environment suggested by XANES.
▪All techniques point to the same Zn(H2O)62+ solvation environment.

