Scientific Achievement
- Electrochemical cycling induces irreversible changes to the oxide structure such as Li/O evolution and crystal rearrangements
- In-situ electron-beam irradiation provides time- and position-sensitive atomic-resolution tracking ability of these transitions to aid in understanding of electrochemical cycling-induced material failures
Significance and Impact
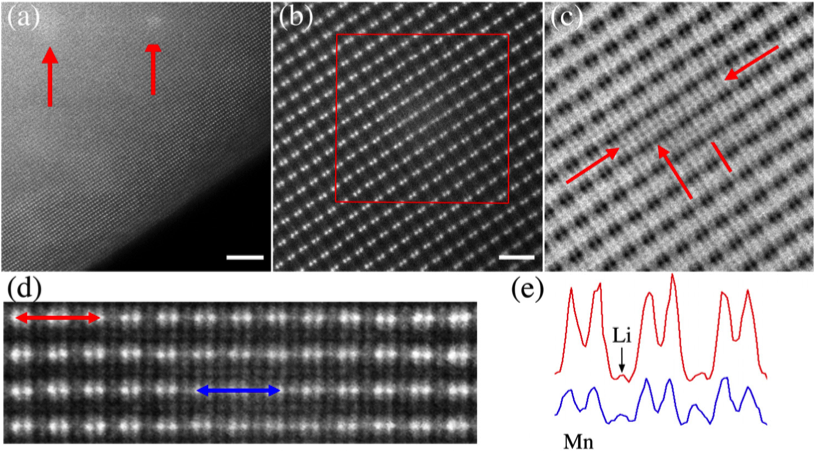
- Irradiation induces local pockets of deformation characterized initially by the creation of Li vacancies and the significant motion of Mn into Li sites; Mn valence > 4+
- Further irradiation leads to structural instability, spinel-like formation, O loss, and a Mn valence near 2+
Research Details
- Analysis can be extended to compare these irradiation results to electrochemically cycled material
- Extensions to multivalent intercalation compounds is proposed
Work performed at University of Illinois at Chicago (JCESR partner) and Argonne National Laboratory (JCESR managing partner) by PJ Phillips, H Iddir, DP Abraham and RF Klie, Applied Physics Letters, 2014.
DOI: 10.1063/1.4896264

